Difference Between Aldol Condensation and Cannizzaro Reaction
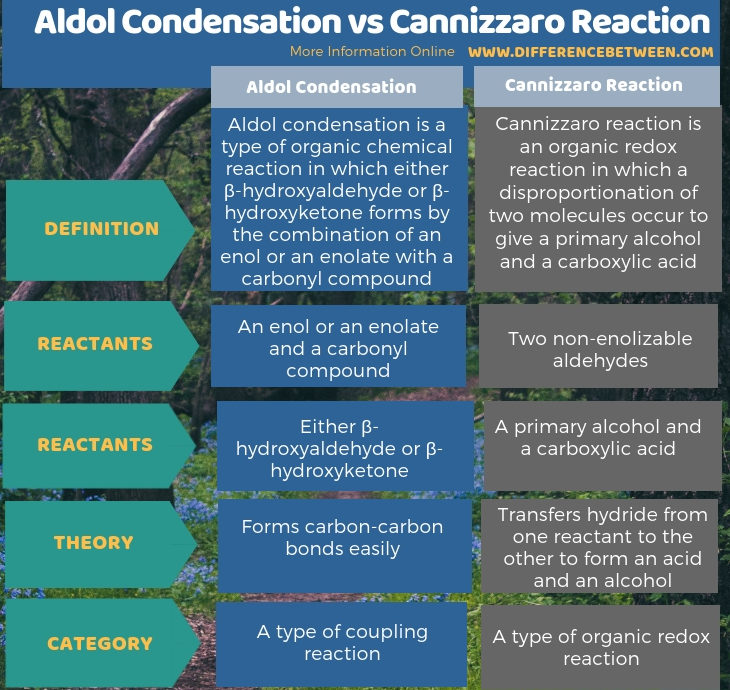
The key difference between aldol condensation and Cannizzaro reaction is that aldol condensation is a type of coupling reaction, whereas Cannizzaro reaction is a type of organic redox reaction.
In an aldol reaction, an enol or an enolate combines with a carbonyl compound to form either β-hydroxyaldehyde or β-hydroxyketone. We call it a coupling reaction because two compounds couple to form one large compound. On the other hand, Cannizzaro reaction is a redox reaction in which one aldehyde molecule undergoes oxidation to form an acid while the other aldehyde undergoes reduction to form an alcohol.
CONTENTS
1. Overview and Key Difference
2. What is Aldol Condensation
3. What is Cannizzaro Reaction
4. Side by Side Comparison – Aldol Condensation vs Cannizzaro Reaction in Tabular Form
5. Summary
What is Aldol Condensation?
Aldol condensation is a type of organic chemical reaction in which either β-hydroxyaldehyde or β-hydroxyketone is formed by the combination of an enol or an enolate with a carbonyl compound. Therefore, we can categorize it as a coupling reaction. The reaction is followed by a dehydration reaction, which gives a conjugated enone. The general reaction is as follows;
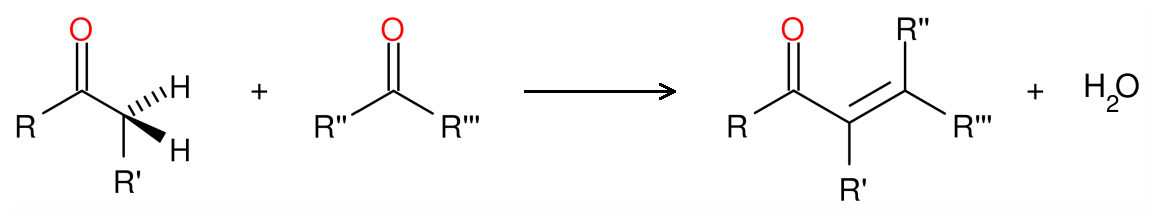
Mechanism
There are two steps: aldol reaction and dehydration reaction. Sometimes, there is a dicarboxylic reaction, as well. The dehydration of the aldol product can occur in two ways: strong base-catalyzed mechanism or acid-catalyzed mechanism. The mechanism of aldol reaction that is catalyzed by a base is as follows:
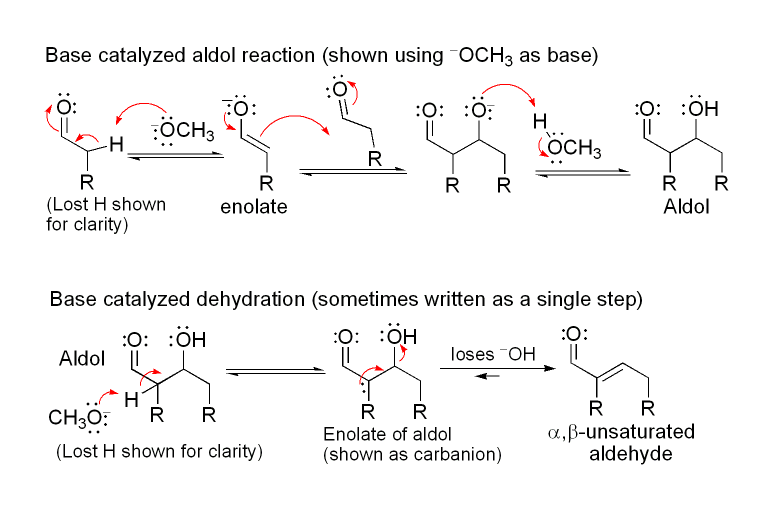
Aldol condensation process is very important in organic synthesis processes. It is because this reaction is a good way to form a carbon-carbon bond.
What is Cannizzaro Reaction?
Cannizzaro reaction is an organic redox reaction in which a disproportionation of two molecules occurs to give a primary alcohol and a carboxylic acid. In this reaction, the disproportion is a base-induced reaction. The reaction is as follows:
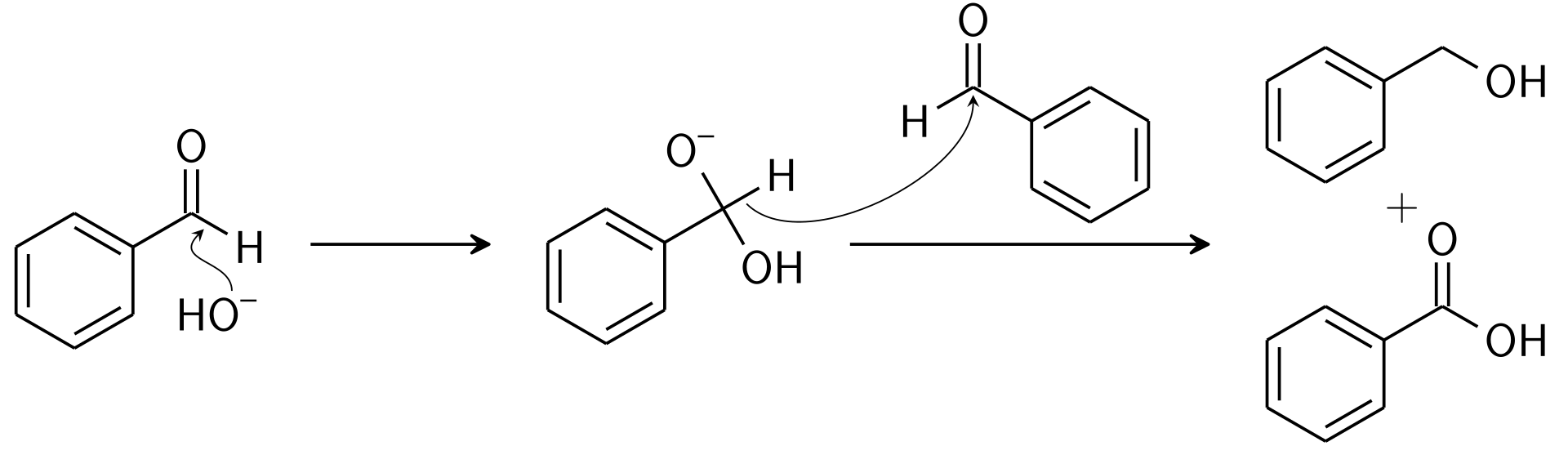
Here, this reaction involves the transfer of a hydride from one substrate to the other. Further, one aldehyde compound undergoes oxidation, and the other one undergoes reduction. Oxidation of aldehyde gives the acid and reduction gives the alcohol.
Mechanism
The mechanism includes nucleophilic acyl substitution on an aldehyde compound. Here, the leaving group concurrently attacks the other aldehyde. When considering the overall reaction, it follows third-order kinetics. The mechanism is as follows:
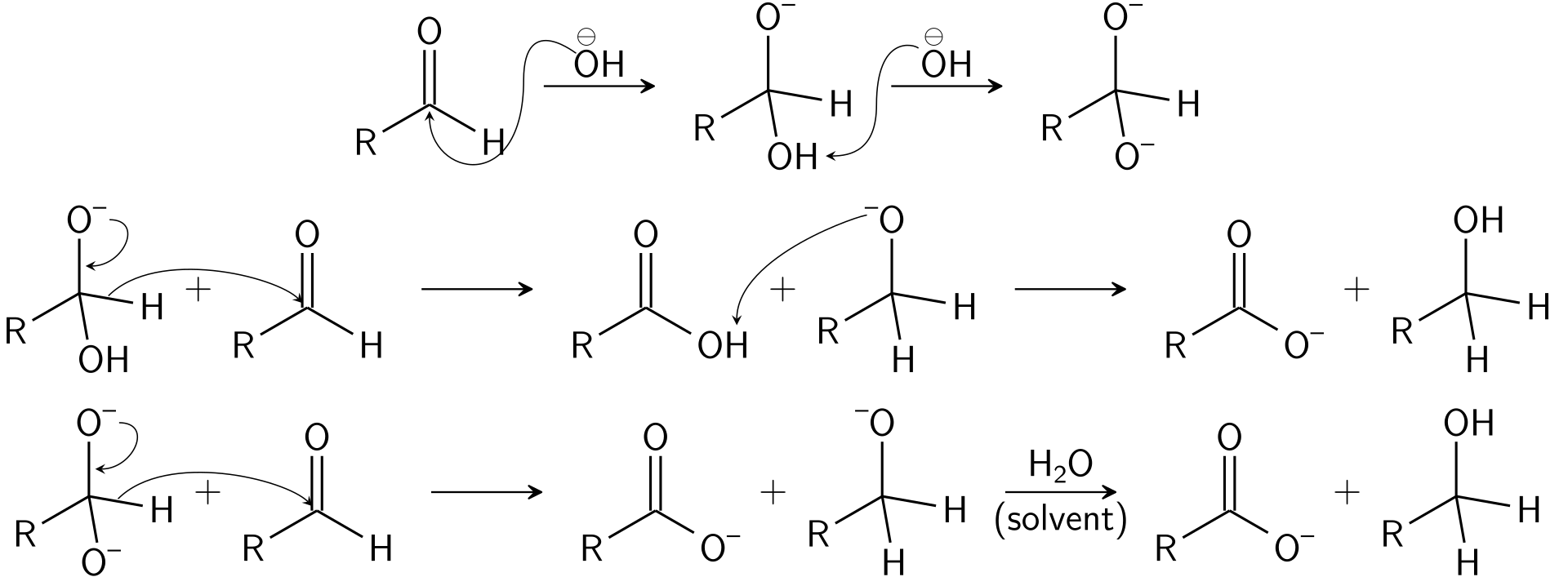
What is the Difference Between Aldol Condensation and Cannizzaro Reaction?
Aldol condensation is a type of organic chemical reaction in which either β-hydroxyaldehyde or β-hydroxyketone forms by the combination of an enol or an enolate with a carbonyl compound. In contrast, Cannizzaro reaction is an organic redox reaction in which a disproportionation of two molecules occurs to give a primary alcohol and a carboxylic acid. So, the key difference between aldol condensation and Cannizzaro reaction is that aldol condensation is a type of coupling reaction, whereas Cannizzaro reaction is a type of organic redox reaction.
Further, the reactants of aldol condensation are an enol or an enolate and a carbonyl compound while for cannizzaro reaction, the reactants are two non-enolizable aldehydes. The products given by aldol condensation are either β-hydroxyaldehyde or β-hydroxyketone, depending on the type of carbonyl compounds that involves as the reactant. But in Cannizzaro reaction, the products are a primary alcohol and a carboxylic acid. Therefore, this is also a difference between aldol condensation and Cannizzaro reaction.
Summary – Aldol Condensation vs Cannizzaro Reaction
Aldol condensation is a type of organic chemical reaction in which either β-hydroxyaldehyde or β-hydroxyketone forms by the combination of an enol or an enolate with a carbonyl compound. Cannizzaro reaction is an organic redox reaction in which a disproportionation of two molecules occurs to give a primary alcohol and a carboxylic acid. The key difference between aldol condensation and Cannizzaro reaction is that the aldol condensation is a type of coupling reaction, whereas the Cannizzaro reaction is a type of organic redox reaction.
Reference:
1. “Aldol Condensation.” Chemistry LibreTexts, Libretexts, 5 June 2019, Available here.
Image Courtesy:
1. “Condensationaldoliqu” By I, Pansanel (CC BY-SA 3.0) via Commons Wikimedia
2. “Enolate aldol mechanism” By Walkerma at en.wikipedia – Transferred from en.wikipedia to Commons by User: Ronhjones using CommonsHelper (Public Domain) via Commons Wikimedia
3. “Cannizzaro reaction-benzaldehyde” By Krishnavedala – Own work, Public Domain) via Commons Wikimedia
4. “Cannizzaro reaction mechanism” By Krishnavedala – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26ty52mpWWTpLulsc2smK2hn6N6orrDZpqapp6ex7ut0ahkq52RmMGqu81o