Difference Between Alkylation and Acylation
Key Difference – Alkylation vs Acylation
Alkylation and acylation are two electrophilic substitution reactions in Organic chemistry. The key difference between alkylation and acylation is the group involved in the substitution process. An alkyl group is substituted in the process of alkylation whereas an acyl group is substituted to another compound in acylation. When this substitution occurs in a benzene ring under catalytic conditions, it is called “Friedel- crafts acylation/alkylation.”
What is Alkylation?
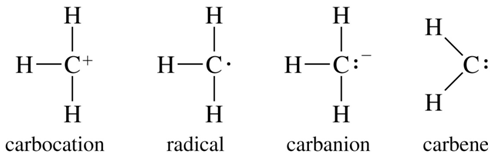
Transferring of an alkyl group from one molecule to another molecule is known as ‘alkylation.’ The transferred alkyl group can be an alkyl carbocation, a free radical, a carbanion, or a carbine. Alkyl group is a part of a molecule having the general formula of CnH2n+1 (n– is an integer, it is equivalent to the number of Carbon in the alkyl group).
What is Acylation?
The process of adding an acyl group to a chemical compound is known as acylation. Acylating agent is the chemical compound which provides the acyl group in this process. The examples of acylating agents are; acyl halides, acetyl chlorides.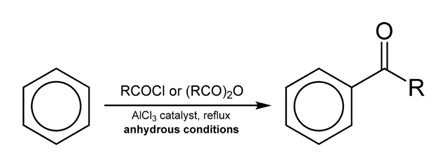
What is the difference between Alkylation and Acylation?
Definition of Alkylation and Acylation:
Alkylation: Alkylation is the transferring of an alkyl group from one molecule to another molecule.
Acylation: Acylation is the process of adding an acyl group to a chemical compound.
Agents:
Alkylation:
The examples of alkylating agents are;
- Alkyl carbocations
- Free radical
- Carbanions
- Carbines
Acylation:
Acyl halides are most commonly used as acylating agents; they are very strong electrophiles when treated with some metal catalysts.
- Acyl halides:
Ethanoil chloride CH3-CO-Cl
- Acyl anhydrides of Carboxylic acids
Alkylation and Acylation Mechanism:
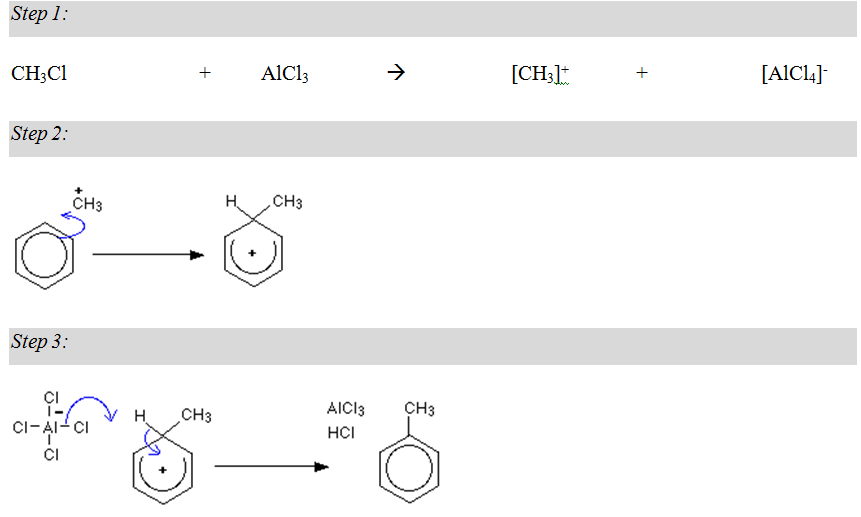
Alkylation:
Alkylation of benzene: In this reaction, a hydrogen atom in the benzene ring is replaced by a methyl group.
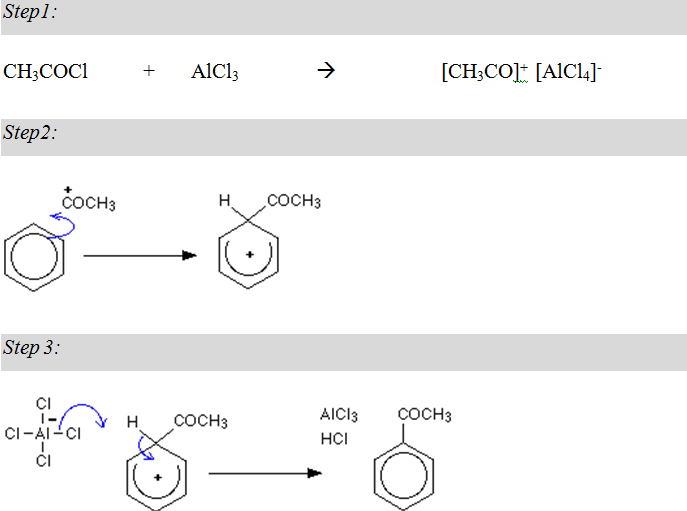
Acylation:
Acylation of Benzene: In this reaction, a Hydrogen atom in the benzene ring is replaced by a CH3CO- group.
 Applications of Alkylation and Acylation:
Applications of Alkylation and Acylation:
Alkylation:
In oil refining process: The alkylation of isobutene with olefins is used to upgrade petroleum. It produces synthetic alkylates having C7-C8 chains. Those are used as premium blending stock for gasoline.
In medicine: A drug class called “alkylating antineoplastic agents” is used in the alkylation process in chemotherapy applications. This is done by the alkylation of DNA with the drug to damage the DNA of cancer cells.
Acylation:
In Biology:
Protein acylation: Post–translational modification of proteins is done by attaching functional groups through acyl linkages.
Fatty acylation: It is the process of adding fatty acids to particular amino acids (myristoylation or palmitoylation).
Limitations of Alkylation and Acylation:
Alkylation:
- When halides are used in alkylation, it must be an alkyl halide. Vinyl or aryl halides cannot be used since their intermediate carbocations are not very stable.
- This reaction involves a carbocation rearrangement process, and a different product will form.
- Poly-alkylation: Attaching more than one alkyl group to the ring. This can be controlled by adding an excessive amount of benzene.
Acylation:
- The acylation only produces ketones. It’s due to the decomposition of HOCl to CO and HCl under the provided reaction conditions.
- Only the activated benzenes are reactive in acylation. In this case, benzenes should be reactive than a mono-halobenzene.
- When aryl amine groups are present, the Lewis acid catalyst (AlCl3) can form a complex making them very un-reactive.
- When amine and alcohol groups are present, they can give N or O acylations instead of the required ring acylation.
Definition of Acyl Group:
A functional group containing a double bonded oxygen atom and an alkyl group to a Carbon atom (R-C=O). In organic chemistry, acid groups are usually derived from carboxylic acids. Aldehydes, ketones and esters also contain acyl groups.
References: Hunt, I. (2016). Reactions of Arenes Electrophilic Aromatic Substitution . Retrieved 7 April, 2016, from here Britannicacom. (2016). Encyclopedia Britannica. Retrieved 7 April, 2016, from herencG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26ty6SwpZmknryvecCnm2auo2KupMXLmquip55k