Difference Between Atomic and Molecular Elements
The key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element.
Atomic elements are the most stable chemical elements; mainly the noble gases. Therefore, they can exist as independent atoms. But they can undergo chemical reactions to form compounds as well. In contrast, molecular elements are chemical compounds containing at least two atoms of the same chemical element. These atoms bind with each other via the formation of chemical bonds between the atoms.
CONTENTS
1. Overview and Key Difference
2. What are Atomic Elements
3. What are Molecular Elements
4. Side by Side Comparison – Atomic vs Molecular Elements in Tabular Form
5. Summary
What are Atomic Elements?
Atomic elements are the chemical species that can exist as independent atoms. This is due to their high stability. There is only one atom in the chemical formula of these elements. Therefore, there are no numerical subscripts with the symbol of atomic elements.
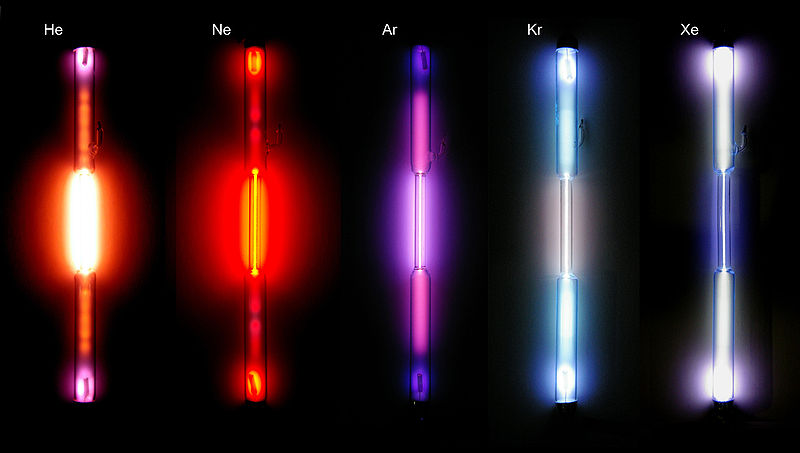
Figure 01: Different Noble Gases
Noble Gases
Noble gases are the group 8 elements of the periodic table that has complete electron configurations. Due to this complete electron configuration, these atoms can exist as individual atoms without forming any chemical bond. But with provided specific conditions, they can form chemical bonds. The noble gases that we know are Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Ra).
What are Molecular Elements?
Molecular elements are the chemical species that has at least two atoms of the same chemical element bonded to each other via chemical bonding. These are different from chemical compounds because a chemical compound contains two or more atoms of different chemical elements.
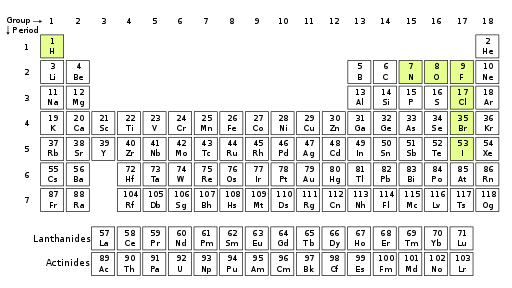
Figure 01: Chemical elements capable of forming diatomic molecules with two atoms of the same Chemical Element.
Moreover, the chemical formula of molecular elements contains one chemical symbol with a numerical subscript indicating the number of atoms present in the molecule some common examples include O2, H2, N2, Cl2, etc.
What is the Difference Between Atomic and Molecular Elements?
Atomic and molecular elements, both terms describe the presence of a single type of chemical element in those chemical species. However, the key difference between atomic and molecular elements is that an atomic element is a chemical species of atoms which contains the same number of protons in the atomic nuclei whereas a molecular element is a molecular substance which consists of a single element. When considering their chemical formulas, we can identify a difference between atomic and molecular elements. That is, the atomic element has only one chemical symbol with no numerical subscripts while the molecular elements have one chemical symbol with a numerical subscript.
The below infographic presents the difference between atomic and molecular elements in tabular form.
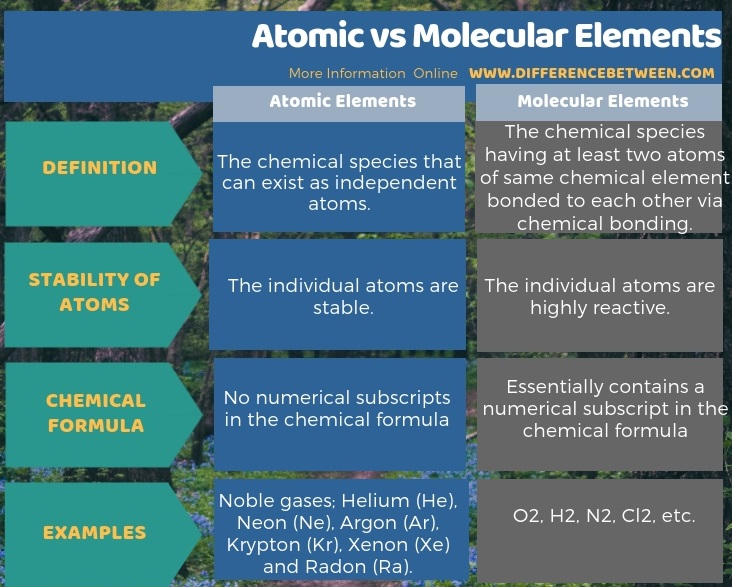
Summary – Atomic vs Molecular Elements
Both terms, atomic and molecular elements, describe the chemical species that has a single type of chemical element rather than different chemical elements. The key difference between atomic and molecular element is that an atomic element is a chemical species of atoms that contains the same number of protons in the atomic nuclei whereas a molecular element is a molecular substance that consists of a single element.
Reference:
1. Libretexts. “5.4: A Molecular View of Elements and Compounds.” Chemistry LibreTexts, Libretexts, 4 May 2018. Available here
2. “Molecules and Atoms.” Phosphorus Properties. Available here
Image Courtesy:
1.Edelgase in Entladungsroehren”By Alchemist-hp – Own work, (CC BY-SA 2.0 de) via Commons Wikimedia
2.”Diatomic molecules periodic table”By White_periodic_table.svg (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26t06ikoptdlrulecyoo56bpaGus3nEpZymnZ6pwHA%3D