Difference Between Axial and Equatorial Position
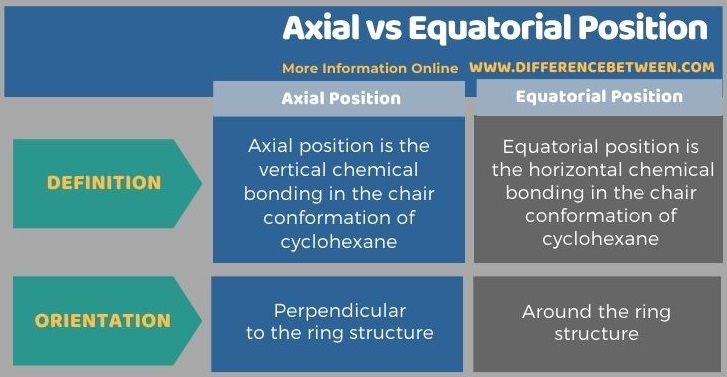
The key difference between axial and equatorial position is that axial bonds are vertical while equatorial bonds are horizontal.
The terms axial and equatorial are important in showing the actual 3D positioning of the chemical bonds in a chair conformation cyclohexane molecule. A conformation is a shape a molecule can take due to the rotation around one or more of its bonds. The bonds are positioned in axial and equatorial positions in order to minimize the angle strain.
CONTENTS
1. Overview and Key Difference
2. What is Chair Conformation
3. What is Axial Position
4. What is Equatorial Position
5. Side by Side Comparison – Axial vs Equatorial Position in Tabular Form
6. Summary
What is Chair Conformation?
Chair conformation is the most stable structure of cyclohexane due to the low energy level of this structure. Typically, all the molecules of cyclohexane occur in chair conformation at room temperature (around 25°C). When we consider a mixture of different structures of the same compound (cyclohexane) at room temperature, we can observe that around 99.99% of the molecules convert into chair conformation. Moreover, when considering the symmetry of this molecule, we can name it as D3d. Here, all the carbon centres are equivalent.
What is Axial Position?
Axial position is the vertical chemical bonding in the chair conformation of cyclohexane. Due to the minimized steric hindrance, the chair conformation is the most stable structure for the cyclohexane molecule. The axial position is perpendicular to the plane of the ring of cyclohexane. Therefore, we can define it as a vertical chemical bond. The bond angle of this type of chemical bonds is usually 90 degrees. More importantly, we can observe axial positions next to each other (in opposite directions).
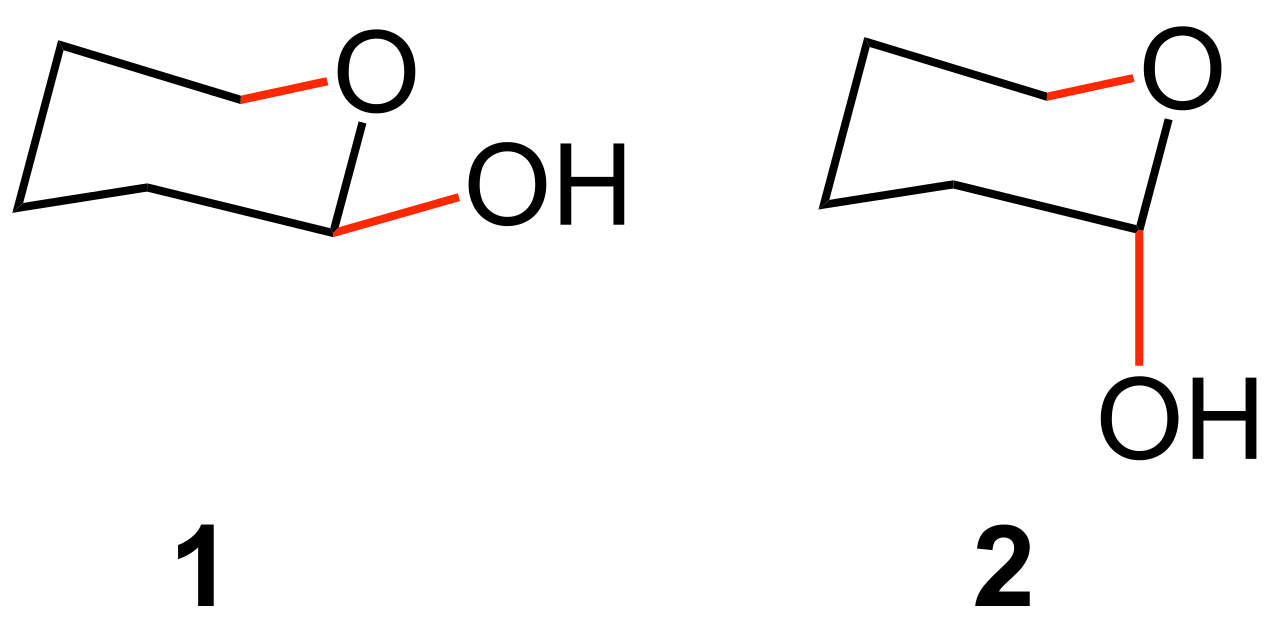
Figure 01: (1) Equatorial Position and (2) Axial Position
What is Equatorial Position
Equatorial position is the horizontal chemical bonding in the chair conformation of cyclohexane. We can find this type of chemical bonding in the chair conformation of cyclohexane. Due to the minimized steric hindrance, the chair conformation is the most stable structure for the cyclohexane molecule.

Figure 02: Chemical Bond in the Equatorial Position
The equatorial position of the cyclohexane molecule can be observed around the ring structure. The name “equatorial” is given to these bonds with the meaning of “bonds that are radiating away from the equator of the ring”. More importantly, we can observe equatorial positions next to each other (in opposite directions).
What is the Difference Between Axial and Equatorial Position?
The terms axial position and equatorial position are discussed under the chair conformation structure of cyclohexane molecule. The key difference between axial and equatorial position is that axial bonds are vertical while equatorial bonds are horizontal. In other words, axial chemical bonds are perpendicular to the ring structure of the cyclohexane molecule while the equatorial positions are around the ring structure, oriented away from the equator of the ring.
Summary – Axial vs Equatorial Position
The terms axial position and equatorial position are discussed under the chair conformation structures of organic chemistry. Chemical bonds in axial position are perpendicular to the ring structure. But the chemical bonds in the equatorial position are around the ring structure that is oriented away from the equator of the ring. The key difference between axial and equatorial position is that axial bonds are vertical while equatorial bonds are horizontal.
Reference:
1. “4.7: Axial and Equatorial Bonds in Cyclohexane.” Chemistry LibreTexts, Libretexts, 14 July 2020, Available here.
2. “Axial and Equatorial: Facts, Summary & Definition: Chemistry Revision.” A Level Chemistry, Available here.
Image Courtesy:
1. “Antiperiplanar vs. synclinal V.2” Von Jü – Eigenes Werk (CC0) via Commons Wikimedia
2. “Tert-butylcyclohexane equatorial” By Keministi – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26t16KYpWWRo7FusdCumK2nop6urXnPqKqirJmku3A%3D