Difference Between Betadine and Iodine
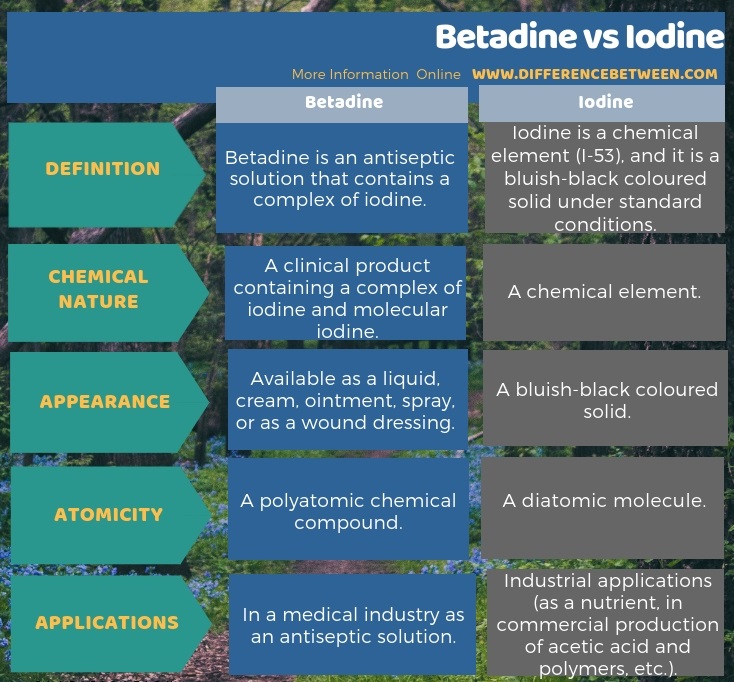
The key difference between betadine and iodine is that the betadine is a clinical product, which mainly contains a complex of iodine and molecular iodine whereas the iodine is a chemical element.
The difference between Betadine and Iodine, basically, stems from their chemical nature. Iodine is a rare chemical element that usually exists as a diatomic molecule. Betadine is a complex chemical compound containing iodine in a complex form. Both iodine and betadine have numerous commercial uses and unique applications; basically, betadine is useful as an antiseptic solution.
CONTENTS
1. Overview and Key Difference
2. What is Betadine
3. What is Iodine
4. Side by Side Comparison – Betadine vs Iodine in Tabular Form
5. Summary
What is Betadine?
Betadine is an antiseptic solution that contains a complex of iodine. It was introduced in the 1960s, and it has wide use as iodophor in modern clinical applications. Furthermore, Povidone-iodine (PVP-Iodine) is the active substance in Betadine; it is a complex of polyvinylpyrrolidone (povidone or PVP).
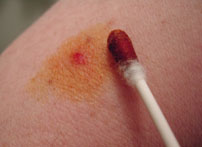
Figure 01: Using Betadine as an Antiseptic
In addition to PVP, molecular iodine (9.0% to 12.0%) is also present in Betadine. i.e., 100 ml of Betadine solution contains about 10 g of Povidone-iodine. Also, it is now available in different formulas such as solution, cream, ointment, spray, and wound dressings.
What is Iodine?
Iodine is a chemical element (I-53), and it is a bluish-black coloured solid under standard conditions. It exists as a diatomic molecule (I2) having only one stable isotope. Furthermore, it occurs in the form of iodine ions in the seawater, fish, oysters, and in some seaweeds. It also occurs in vegetables grown in iodine-rich soil and in dairy products.
The word Iodine is a Greek word, meaning purple or violet. People used iodine for more than 170 years as a highly effective antimicrobial agent in clinical treatments. Therefore, iodine is dark violet, the non-metallic natural liquid that plays an important role in human metabolism. Accordingly, iodine is an essential element in the production of thyroid hormones. Hence, iodine deficiency can result in hypothyroidism. Also, we consider it as the most effective disinfectant available.

Figure 02: Appearance of Iodine
Moreover, the use of iodine is safe for several reasons. When iodine makes a bond with another molecule, it becomes less toxic and, in a single application, iodine slowly releases from the reservoir career molecule over a sustained period instead of high concentrations at once.
What is the Difference Between Betadine and Iodine?
Betadine is an antiseptic solution that contains a complex of iodine whereas iodine is a chemical element (I-53) and it is a bluish-black coloured solid under standard conditions. Therefore, Iodine is a chemical element, and Betadine is a clinical product, which mainly contains a complex of iodine and molecular iodine. Thus, this is the key difference between betadine and iodine. When considering the applications of each of them, we use betadine mostly in the medical industry as an antiseptic solution, but iodine has so many industrial applications (as a nutrient, in commercial production of acetic acid and polymers, etc.). Also, there exists a difference between betadine and iodine based on the atomicity. Iodine is a diatomic molecule whereas betadine is a polyatomic chemical compound.
Summary – Betadine vs Iodine
In summary, betadine is a complex compound containing iodine as a major component. The key difference between betadine and iodine is that the betadine is a clinical product, which mainly contains a complex of iodine and molecular iodine whereas iodine is a chemical element.
Reference:
1. “Iodine.” Wikipedia, Wikimedia Foundation, 4 Oct. 2018. Available here
2. “Povidone-Iodine.” Wikipedia, Wikimedia Foundation, 4 Oct. 2018. Available here
Image Courtesy:
1.”ExAntiseptic” (Public Domain) via Commons Wikimedia
2.”Sample of iodine”By LHcheM – Own work, (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26uxK2YnaGemnqiusNmraxlmaSxqrrEaA%3D%3D