Difference Between Binary Acids and Polyatomic Acids
The key difference between binary acids and polyatomic acids is that binary acids contain atoms from only two different chemical elements, whereas polyatomic acids contain atoms from two or more different chemical elements.
An acid is an inorganic chemical compound that can neutralize an alkaline substance. Acids are able to dissolve most metals. We can easily identify an acid using litmus paper – blue litmus changes into red colour upon soaking it with an acid. There are different types of acids; binary acids and polyatomic acids are two such types.
CONTENTS
1. Overview and Key Difference
2. What are Binary Acids
3. What are Polyatomic Acids
4. Side by Side Comparison – Binary Acids vs Polyatomic Acids in Tabular Form
5. Summary
What are Binary Acids?
Binary acids are inorganic substances having hydrogen-bonded to another chemical element. This second chemical element is mostly a nonmetallic element. The term “binary” refers to a substance having “two” components of something; in this context, it is two different chemical elements. The acidity of these substances arises due to their ability to release hydrogen as a cation or proton, which causes the acidity of its aqueous solution. The most common binary acids include hydrofluoric acid (HF), hydrochloric acid (HCl), and hydrobromide acid (HBr). Moreover, binary acids may have one or more hydrogen atoms per molecule, depending on the valency of the nonmetal that is bonded to the hydrogen atom(s), e.g. H2S.
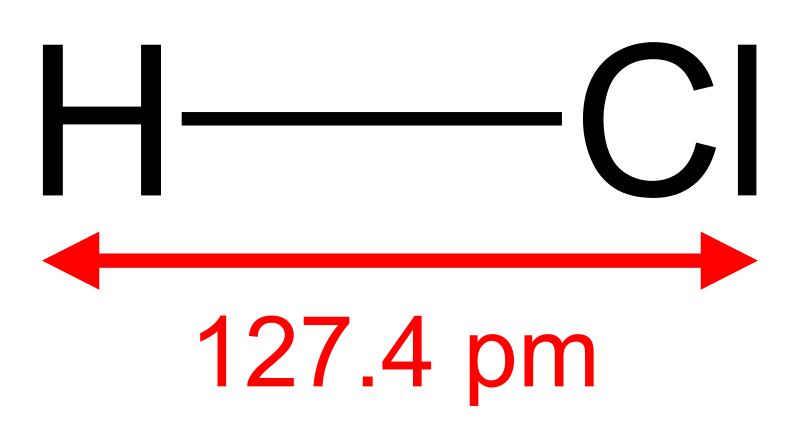
Figure 01: Hydrogen Chloride
Binary acids can be strong acids, weak acids or moderately acidic. This acidic strength depends on the strength of the covalent bond between a hydrogen atom and a nonmetallic atom. Since all the binary acids contain hydrogen atoms, the name of the binary acid starts with “hydro-”.
What are Polyatomic Acids?
Polyatomic acids are inorganic compounds containing atoms with two or more different chemical elements. However, the ions that form from the dissociation of a polyatomic acid can be either monoatomic or polyatomic because some polyatomic acids have only two different chemical elements and the removal of hydrogen atom forms a monoatomic ion.
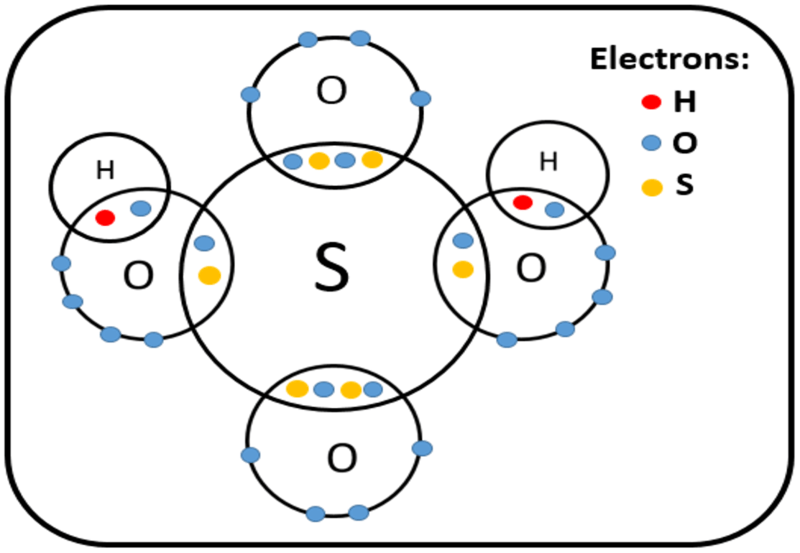
Figure 02: Structure of Sulfuric Acid
Some common examples of polyatomic acids include carbonic acid (H2CO3), sulfuric acid (H2SO4), sulfurous acid (H2SO3), nitric acid (HNO3), etc.
What is the Difference Between Binary Acids and Polyatomic Acids?
An acid is a substance that can neutralize an alkaline substance. The key difference between binary acids and polyatomic acids is that binary acids contain atoms from only two different chemical elements, whereas polyatomic acids contain atoms from two or more different chemical elements.
Moreover, binary acids always form a monoatomic conjugate base, while polyatomic acids may form a monoatomic conjugate base or a polyatomic base. Also, binary acids are mostly strong to moderate acids. Hydrofluoric acid (HF), hydrochloric acid (HCl), and hydrobromide acid (HBr) are some examples of binary acids. Polyatomic acids, on the other hand, can be strong acids, weak acids or moderately acidic compounds. Some examples include carbonic acid (H2CO3), sulfuric acid (H2SO4), and nitric acid (HNO3).
Below infographic tabulates side-by-side the differences between binary acids and polyatomic acids.
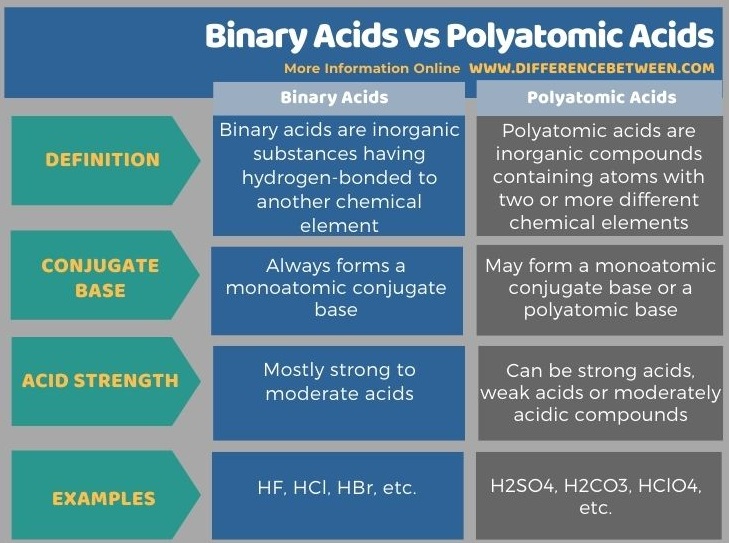
Summary – Binary Acids vs Polyatomic Acids
We can easily identify acids using litmus papers; blue litmus turns red when soaked with an acid. There are different types of acids, such as binary acids and polyatomic acids. The key difference between binary acids and polyatomic acids is that binary acids contain atoms from only two different chemical elements, whereas polyatomic acids contain atoms from two or more different chemical elements.
Reference:
1. Helmenstine, Anne Marie. “What Are Acids and Bases?” ThoughtCo, Aug. 26, 2020, Available here.
2. “5.9: Naming Acids.” Chemistry LibreTexts, Libretexts, 20 Nov. 2020, Available here.
Image Courtesy:
1. “Hydrogen-chloride-2D-dimensions” (Public Domain) via Commons Wikimedia
2. “H2so4 pic” By Jadder1224 – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26uyKeYq7FdlrCqsNJmmKecXaW8rcXAraamoZNirqS1w6xm