Difference Between Carbonic Acid and Carbolic Acid
The key difference between carbonic acid and carbolic acid is that carbonic acid is a carboxylic acid compound, whereas carbolic acid is an alcohol.
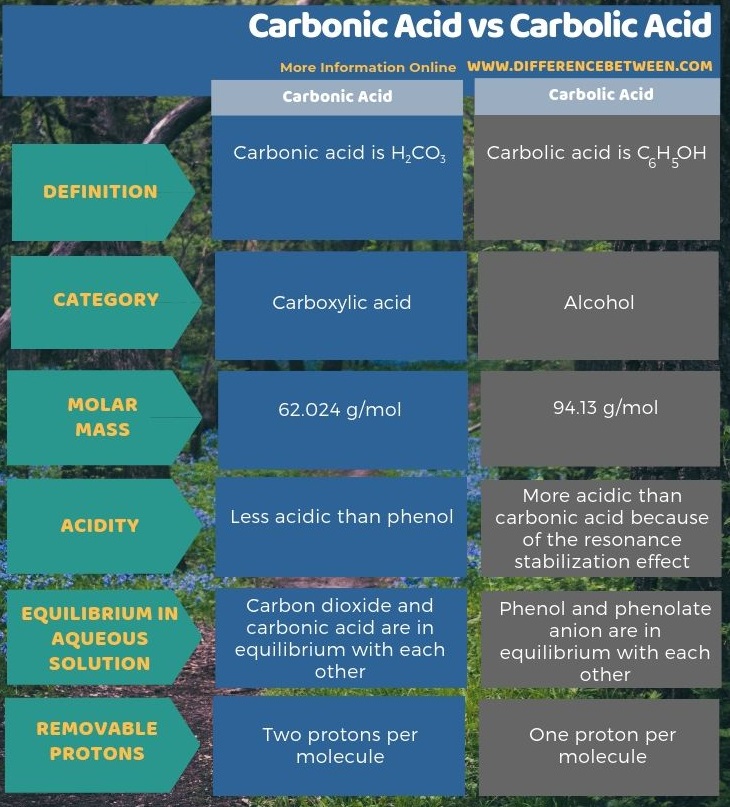
Although the terms carbonic acid and carbolic acid sounds similar, they refer to two different chemical compounds. Carbonic acid is H2CO3 while carbolic acid is C6H5OH. They have different chemical and physical properties.
CONTENTS
1. Overview and Key Difference
2. What is Carbonic Acid
3. What is Carbolic Acid
4. Side by Side Comparison – Carbonic Acid vs Carbolic Acid in Tabular Form
5. Summary
What is Carbonic Acid?
Carbonic acid is H2CO3. Sometimes, we give this name to solutions having carbon dioxide dissolved in water or carbonated water. It is because carbonated water contains a small amount of H2CO3. Further, this is a weak acid, and it can form two types of salts as carbonates and bicarbonates. The molar mass of the compound is 62.024 g/mol.
When carbon dioxide dissolves in water, this compound enters an equilibrium between carbon dioxide and carbonic acid. The equilibrium is as follows;
CO2 + H2O ⟷ H2CO3
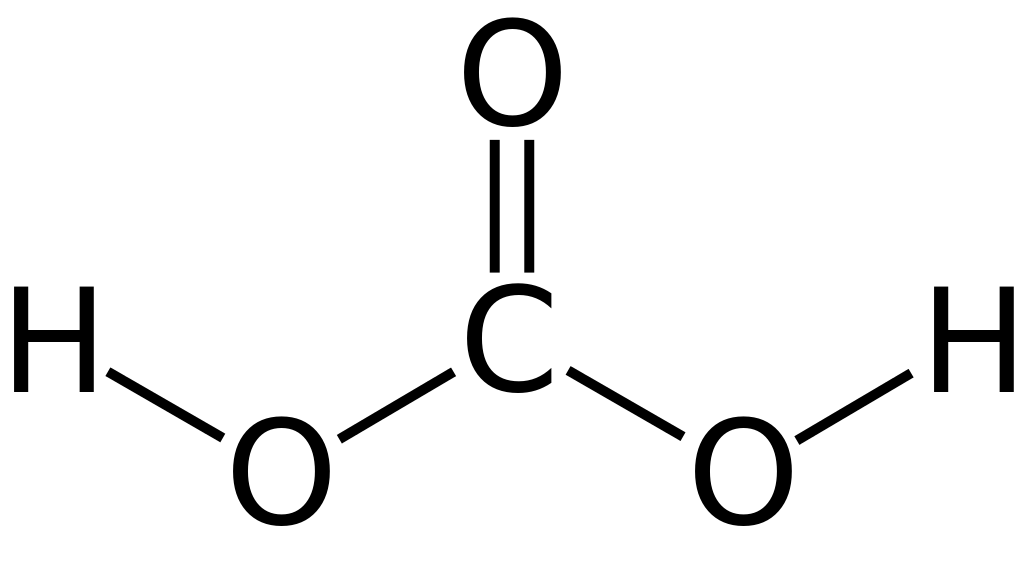
Figure 01: Structure of Carbonic Acid
If we add an excess of carbonic acid to a base, it gives bicarbonate. But, if there is an excess of base, then carbonic acid tends to give carbonated salts. More precisely, carbonic acid is a carboxylic acid compound which has two hydroxyl group substituents attached to the carbonyl carbon. Moreover, it is a polyprotic acid, which is capable of donating protons. It has two removable protons; thus, it is specifically diprotic.
What is Carbolic Acid?
Carbolic acid is C6H5OH. It is an organic compound. The common name of this compound is “phenol”. It has a benzene ring with one of its hydrogen atoms replaced with a hydroxyl group. It occurs as a white crystalline, volatile solid. The acidity of this compound is mild, but we need to take care when handling due to the chemical burns that it may cause. The molar mass of the compound is 94.13 g/mol. It has a sweet odor because it is an aromatic compound.
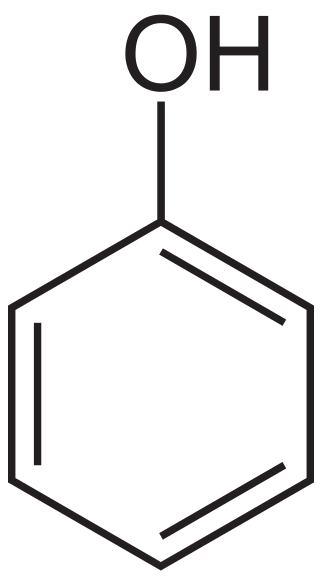
Figure 02: Structure of Carbolic Acid
Moreover, it is a weak acid, and in aqueous solution, it exists in equilibrium with phenolate anions. The pH of this aqueous solution can range from 8 to 12. Due to the resonance stabilization of this compound, phenol is more acidic than the corresponding aliphatic weak acids.
What is the Difference Between Carbonic Acid and Carbolic Acid?
Carbonic acid is H2CO3 while carbolic acid is C6H5OH. The key difference between carbonic acid and carbolic acid is that carbonic acid is a carboxylic acid compound, whereas carbolic acid is an alcohol.
Moreover, a further difference between carbonic acid and carbolic acid is that although both are weak acids, carbolic acid is more acidic than carbonic acid because of the resonance stabilization effect in the compound.
Below infographic shows more comparisons related to the difference between carbonic acid and carbolic acid.
Summary – Carbonic Acid vs Carbolic Acid
Carbonic acid is H2CO3 while carbolic acid is C6H5OH. The key difference between carbonic acid and carbolic acid is that carbonic acid is a carboxylic acid compound, whereas carbolic acid is an alcohol.
Reference:
1. “Phenol.” Wikipedia, Wikimedia Foundation, 10 July 2019, Available here.
Image Courtesy:
1. “Carbonic-acid-2D” By Eetwartti – Own work based on Image:Carbonic-acid-2D.png (Public Domain) via Commons Wikimedia
2. “Phenol2” By NEUROtiker – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vwKuZqKaZmHqir8idZJqmlGKwor7BqKOim12WsKqwjg%3D%3D