Difference Between Catenation and Tetravalency
The key difference between catenation and tetravalency is that catenation includes the binding of atoms of the same chemical element to form chain or ring structures whereas tetravalency refers to the ability to form four covalent bonds.
Both terms catenation and tetravalency are used alongside with the chemical element carbon due to its characteristic properties. Carbon can form chain or ring structures by binding many carbon atoms via covalent bonds and one carbon atom shows a valency of four because it has four valence electrons and it can accept four other electrons to form covalent bonds.
CONTENTS
1. Overview and Key Difference
2. What is Catenation
3. What is Tetravalency
4. Side by Side Comparison – Catenation vs Tetravalency in Tabular Form
5. Summary
What is Catenation?
Catenation refers to the ability of atoms of a particular chemical element to bind to itself, forming chain or ring structures. In catenation, we talk mainly about the chemical element carbon, which is able to form aliphatic and aromatic structures via binding a large number of carbon atoms. In addition, there are some other chemical elements that can form these structures, including sulfur and phosphorous.
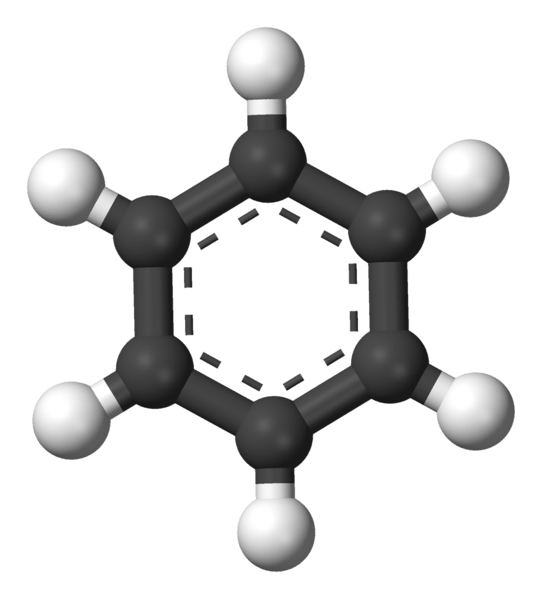
Figure 01: Benzene is Formed from the Catenation of Carbon Atoms
However, if a certain chemical element undergoes catenation, it must have a valency that is at least two. Also, this chemical element must be able to form strong chemical bonds between the atoms of its kind; e.g. covalent bonds. Sometimes, it is referred to as polymerization. Some examples of chemical elements that can undergo catenation are as follows:
What is Tetravalency?
The term tetravalency refers to the ability of an atom of a particular chemical element to form four covalent bonds. In other words, it is the property of having a valency of four, so it is capable of bonding with four other atoms of a different chemical element. In this term, “tetra” means “four”. The most common chemical element with tetravalency is the carbon atom. It has four electrons in its outermost valence shell and it can either donate these four electrons or can accept four electrons from outside. Another example is silicon, which also has four valence electrons and behaves similar to carbon.
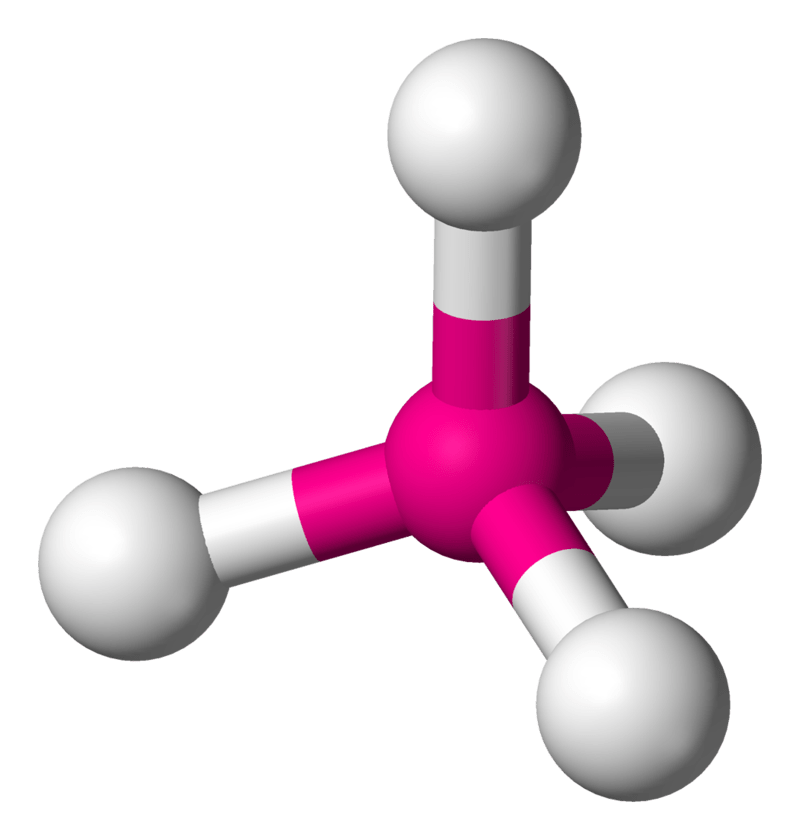
Figure 02: Tetrahedral Geometry
Due to tetravalency, atoms tend to form tetrahedral molecules by accepting four electrons from four different atoms and binding with them through covalent bonds. Based on the type of covalent bond (single covalent bonds, double bonds and triple bonds), the shape and geometry of the molecules formed by these atoms may vary. Eg: if an atom forms two single bonds and one double bond, it gives a trigonal planar molecule and if there are two double bonds, the molecule formed from this tetravalent atom if linear.
What is the Difference Between Catenation and Tetravalency?
The key difference between catenation and tetravalency is that catenation includes the binding of atoms of the same chemical element to form chain or ring structures, whereas tetravalency refers to the ability to form four covalent bonds.
The following table summarizes the difference between catenation and tetravalency.
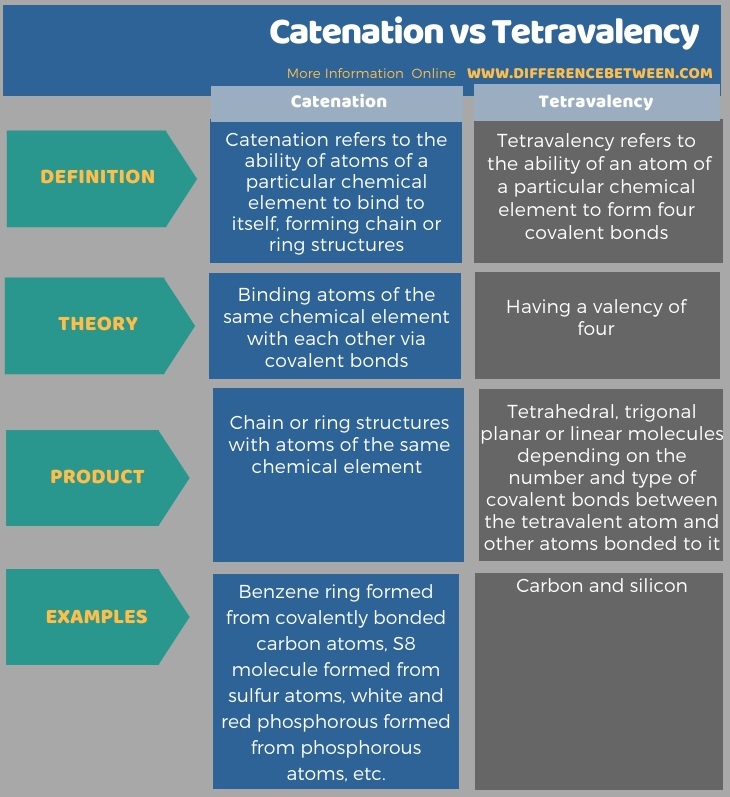
Summary – Catenation vs Tetravalency
Catenation and tetravalency are terms that are mainly used along with the chemical element carbon. The key difference between catenation and tetravalency is that catenation includes the binding of atoms of the same chemical element to form chain or ring structures, whereas tetravalency refers to the ability to form four covalent bonds.
Reference:
1. Helmenstine, Anne Marie. “Catenation Definition and Examples.” ThoughtCo, Feb. 11, 2020, Available here.
2. “Catenation.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 8 Aug. 2007, Available here.
Image Courtesy:
1. “Benzene-aromatic-3D-balls” By Benjah-bmm27 – Own work (Public Domain) via Commons Wikimedia
2. “Tetrahedral-3D-balls” (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vwK2cp5mknryvecCnm2aslam%2FosLApZynm6lk