Difference Between Chelating Agent and Sequestering Agent
Key Difference – Chelating Agent vs Sequestering Agent
Chelating agents and sequestering agents remove metal ions from a solution by forming a complex with that particular metal ion. This process is called chelation. It can be used to remove water hardness or heavy metals from the water. Many chelators and sequestrants have metal ion preferences, which means, the chelator or the sequestrant will bind with a particular metal ion before binding with other metal ions in that system. The key difference between chelating agent and sequestering agent is that a chelating agent can bind with a single metal ion whereas a sequestering agent can bind with few metal ions at a time.
CONTENTS
1. Overview and Key Difference
2. What is a Chelating Agent
3. What is a Sequestering Agent
4. Similarities Between Chelating Agent and Sequestering Agent
5. Side by Side Comparison – Chelating Agent vs Sequestering Agent in Tabular Form
6. Summary
What is a Chelating Agent?
A chelating agent is a substance which has the ability to make several bonds with a single metal ion and form a complex. The metal ion cannot participate in any other reaction that occurs in the system due to the formation of a complex. This is called chelation. If the chelating agent makes two bonds with the metal ions, the chelating agent is called bidentate; if it forms more bonds, it is called multidentate.
The chelating agent forms stable water soluble complexes of metals. This prevents the metal from participating in its normal reactions. Chelating agent forms coordination bonds with the metal ion, changing the chemical structure of metal ion. Chelating agents are very important in chemical reactions. If a solution has a mixture of two metal ions, we can add a chelating agent to prevent the other metal ion from interfering in the reactions in order to find the amount of one metal ion present in the solution. Chelating agents are either natural or synthetic organic compounds used in industrial or biological applications.
A good example of a chelating agent is Ethylenediamine. It can form two bonds with transition metal ions such as Nickel (II). The nickel ion has six covalent electrons; in other words, it has three pairs of electrons. Thus, three Ethylenediamine molecules will bind with one metal ion.
Another common example is EDTA. It is mostly used in soaps and detergents since the EDTA molecule can bind with calcium and magnesium ions in hard water, preventing interferences in the cleaning process of soaps and detergents.
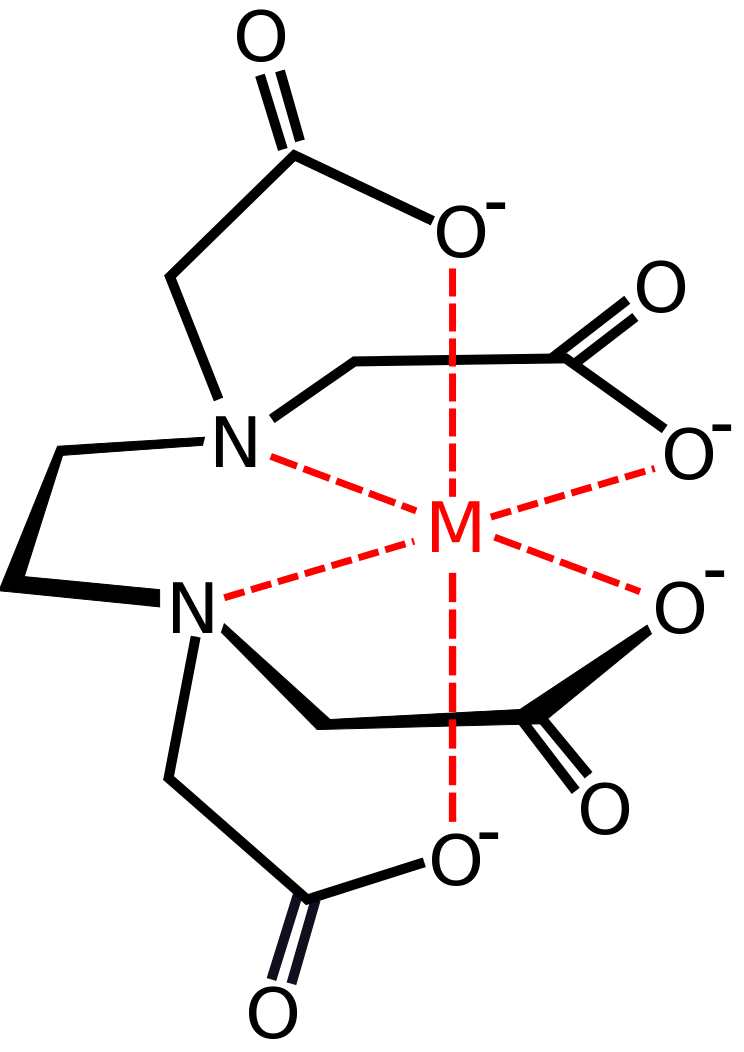
Figure 01: EDTA Binding with a Single Metal Ion
What is a Sequestering Agent?
Sequestering agents are chemical substances which can bind with metal ions in a solution. The combination of sequestering agent and the metal ions forms a stable, water soluble complex. Then that particular metal ion cannot undergo other reactions (reactions which the metal ion undergoes when there is no sequestering agent). Though it shows the same action as a chelating agent, it differs from a chelating agent by its way of “coating” a metal ion; sequestering agents are composed of few active sites which can bind with metal ions. This causes the sequestering agent to be more powerful than a chelating agent since a chelating agent can only bind with a single metal ion.
Most of the times, metal ions resemble a chain arrangement. Then the binding of sequestering agents to the ends of the chains forms a ring-like structure which can easily be removed.
What are the similarities between Chelating Agent and Sequestering Agent?
- Sequestering agents are also a type of chelating agent.
- Both chelating and sequestering agents can bind with metal ions in a solution and can prevent the metal ion from undergoing its normal reactions.
- Chelating agents and sequestering agents are organic compounds which can be either natural or synthetic.
- Both can form stable, water soluble complexes with metal ions.
What is the difference between Chelating Agent and Sequestering Agent?
Chelating Agent vs Sequestering Agent | |
| Chelating agents are chemical compounds that can bind with a metal ion in a solution and prevent it from its normal reactions. | Sequestering agents are chemical compounds that can bind with several metal ions in a solution and prevent it from its normal reactions. |
| Number of Metal Ions | |
| Chelating agents bind with a single metal ion at a time. | Sequestering agents can bind with several metal ions at a time. |
| Active Sites | |
| Chelating agents have one active site per molecule. | Sequestering agents have few active sites per molecule. |
| Potential | |
| Chelating agents are less powerful due to the presence of a single active site. | Sequestering agents are more powerful due to the presence of several active sites. |
| Formation of Complex | |
| Chelating agents form complex molecules which are water soluble. | Sequestering agents form ring-like structures which can be removed from the solution. |
Summary – Chelating Agent vs Sequestering Agent
Chelating agents and sequestering agents are important in industrial, biological and medical applications. It is also useful for getting rid of hardness in water. Although chelating agents and sequestering agents do the same action in a system, they are different terms. The main difference between chelating agent and sequestering agent is that a chelating agent can bind with a single metal ion whereas a sequestering agent can bind with few metal ions at a time.
Download PDF Version of Chelating Agent vs Sequestering Agent
You can download PDF version of this article and use it for offline purposes as per citation notes. Please download PDF version here Difference between Chelating Agent and Sequestering Agent.
References:
1. Tony Hargreaves. “CHELATING AGENTS, SEQUESTRANTS.” CHELATING AGENTS, SEQUESTRANTS | Engineering360. N.p., n.d. Web. Available here. 05 June 2017.
2. Mazadul Hasan. “Sequestering agents.” LinkedIn SlideShare. N.p., 22 May 2014. Web. Available here. 05 June 2017.
Image Courtesy:
1. “Metal-EDTA” By Smokefootderivative work: Chamberlain2007 (talk) – Medta.png, Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vx56jmqyZo7Rurcaepa1lkaOxbsLSZqqeqaWawLWx0aKloGWRnLKvwI4%3D