Difference Between Cl2 and Cl3
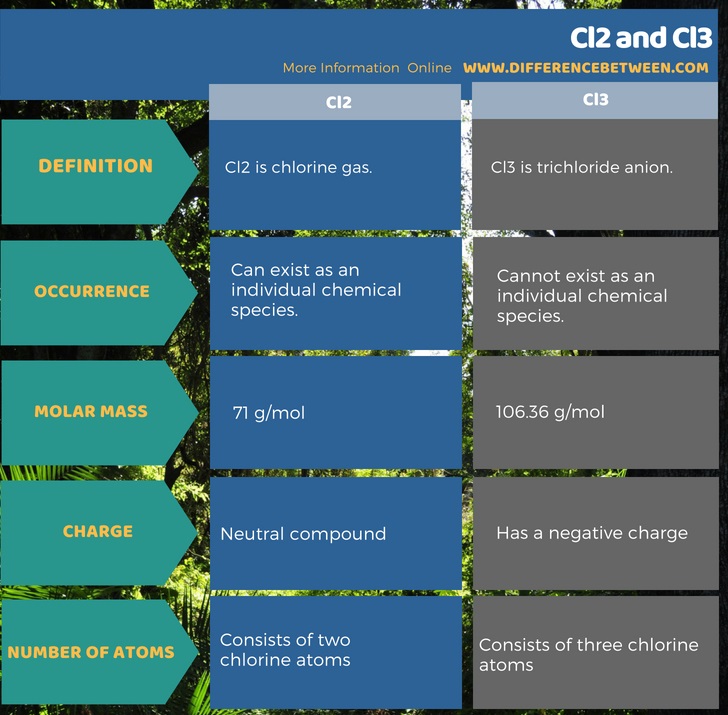
The key difference between Cl2 and Cl3 is that Cl2 is a molecule consisting of two atoms whereas Cl3 is an anion consisting of three atoms. Hence, Cl3 has a negative electrical charge, but Cl2 is neutral.
Cl2 and Cl3 are chemical species containing chlorine atoms. Chlorine is a chemical element having the symbol Cl and atomic number 17. It forms a wide range of compounds in combination with many other metals and nonmetals.
CONTENTS
1. Overview and Key Difference
2. What is Cl2
3. What is Cl3
4. Side by Side Comparison – Cl2 vs Cl3 in Tabular Form
5. Summary
What is Cl2?
Cl2 is the chlorine gas. It is a diatomic, greenish-yellow colored and also has a pungent, suffocating odor. This gas is about 2.5 times heavier than normal air. Although it is a useful gas, we should handle it carefully because it is a strong oxidizing agent. Therefore, it makes this gas a corrosive compound and an irritant to eyes and respiratory system when inhaled. It becomes a liquid at -34◦C. Therefore, it is the boiling point of Cl2. The molar mass of Cl2 is 71 g/mol.

Figure 01: Chlorine Gas
People used chlorine gas in the chemical warfare in the first World War. That is because it can cause suffocation, constriction of the chest, tightness in the throat and also oedema of lungs. However, this gas is useful in water purification, industrial waste sanitation, sanitation of swimming pool water, manufacturing carbon tetrachloride and also for bleaching purposes.
What is Cl3?
Cl3 is an anion of chlorine. We call it trichloride anion. It forms when a chloride anion (Cl–) reacts with a Cl2 molecule. Furthermore, this anion does not exist as an individual chemical species. It always occurs in combination with another chemical element or cation. The molar mass of this chemical species is 106.36 g/mol. This anion ma form in gas phase as in the following chemical reaction.
Cl– + SO2Cl2 ↔ Cl3– + SO2
What is the Difference Between Cl2 and Cl3?
Cl2 is chlorine gas. Cl3 is trichloride anion. Both these consist of chlorine atoms. Not only in their chemical formula, but the difference between Cl2 and Cl3 is also in the occurrence. That is because Cl2 can exist as an individual compound while Cl3 cannot exist itself due to the highly reactive nature. This reactive nature comes from its negative charge.
Summary – Cl2 vs Cl3
Both Cl2 and Cl3 are chemical compounds of chlorine. They differ from each other in their structure and properties such as the reactivity. The difference between Cl2 and Cl3 is that Cl2 is a molecule consist of two atoms whereas Cl3 is an anion consist of three atoms.
Reference:
1. “Chlorine.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine. Available here
2. “Cl3 Anion.” Naphthalene, National Institute of Standards and Technology. Available here
Image Courtesy:
1.’Chlorine ampoule’By W. Oelen (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vy2tkmqaUYrCtf44%3D