Difference Between Classical and Nonclassical Carbocation
The key difference between classical and nonclassical carbocation is that classical carbocations have a carbon atom having six electrons in three chemical bonds, whereas nonclassical carbocations have a three-center two-electron structure.
A carbocation is a chemical species that is a moiety of an organic molecule. It has a positive charge on a carbon atom. A simple example of a carbocation is CH3+. Some carbocations have more than one positive charge, on the same carbon atom or a different atom. Moreover, carbocations are reactive intermediates in organic reactions due to the presence of a positive charge; there are six electrons in a carbon atom, which makes it unstable (presence of eight electrons ensures the stability); therefore it tends to seek electrons.
CONTENTS
1. Overview and Key Difference
2. What is Classical Carbocation
3. What is Nonclassical Carbocation
4. Side by Side Comparison – Classical vs Nonclassical Carbocation in Tabular Form
5. Summary
What is Classical Carbocation?
A classical carbocation is an ion containing a positively charged carbon atom which has six electrons that take part in three chemical bonds. We can name this carbon atom as a three-coordinate positive carbon.
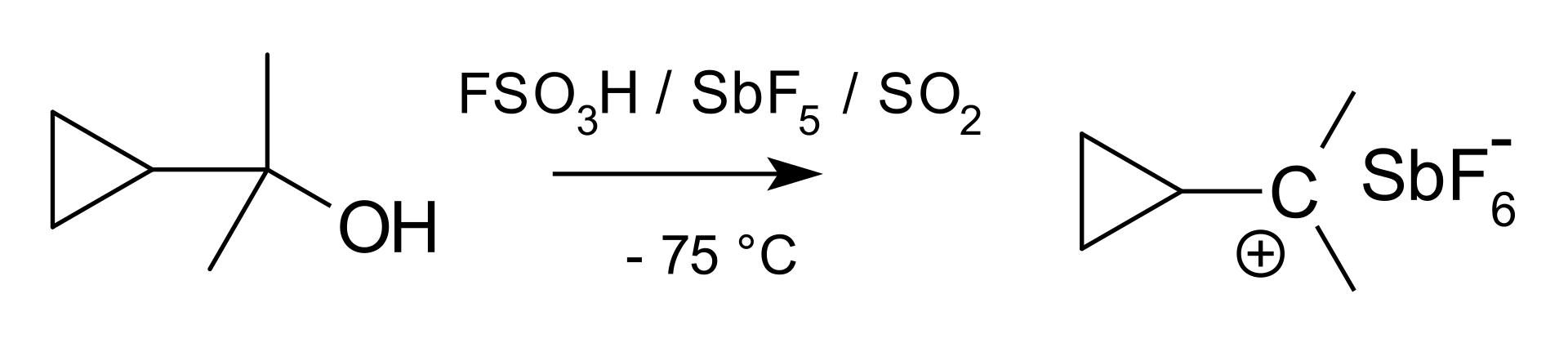
Figure 01: Formation of a Classical Carbocation
To ensure maximum stability, the carbon atom should have eight valence electrons. But in the carbocation, there are only six electrons in the carbon atom having a positive charge. Therefore, it tends to share two more electrons from an electronegative species. This makes the carbon atom stable and neutralizes the positive charge. This is the reason for the high reactivity of classical carbocations. However, the energy of a classical carbocation is low compared to the energy of the corresponding nonclassical carbocation. But this difference in their energies is very small.
What is Nonclassical Carbocation?
A nonclassical carbocation is an ion containing a positively charged carbon in a three-center two-electron center. This means, there are three atoms sharing two electrons in these carbocations. This type of electron sharing is named as delocalization of the electrons.
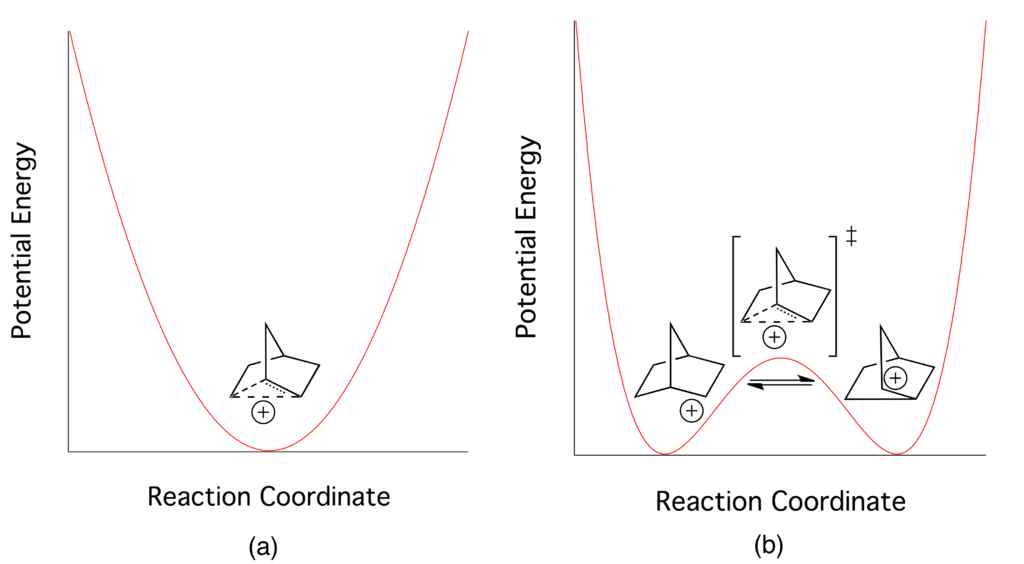
Figure 02: Energy Difference Between Classical and Nonclassical Carbocations
The most common example of a nonclassical carbocation is 2-norbornyl cation. It exists in a less symmetrical three-center two-electron structure. There is very little difference in the energy between classical and nonclassical carbocations. Therefore, it is very difficult to distinguish them experimentally.
What is the Difference Between Classical and Nonclassical Carbocation?
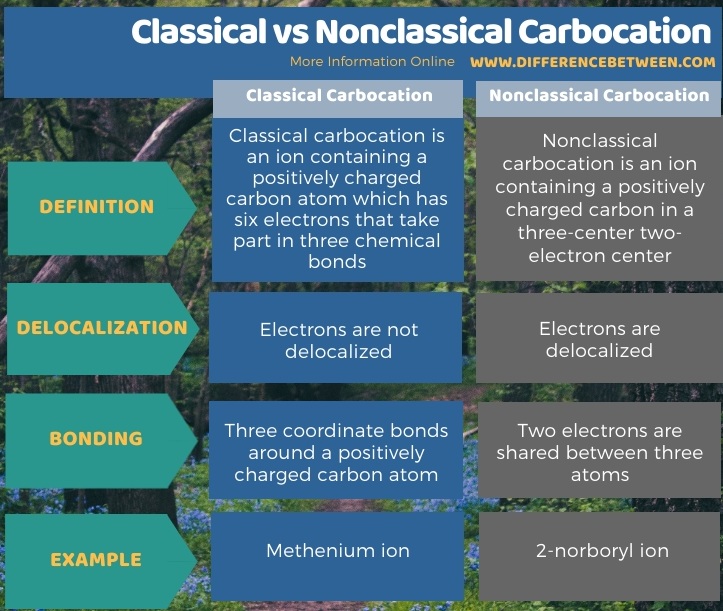
We can classify carbocations into two groups as classical and nonclassical carbocations, depending on the chemical structure. The key difference between classical and nonclassical carbocation is that classical carbocations have a carbon atom having six electrons in three chemical bonds, whereas nonclassical carbocations have three-center two-electron structure. The energy of nonclassical carbocation is higher than the energy of classical carbocation, but the difference between these energies is very small; hence, it is very difficult to distinguish the difference between classical and nonclassical structures.
Moreover, the activation energy for the conversion of the classical carbocation into a nonclassical carbocation or vice versa is very small. In addition to these, classical carbocations have the positive charge on the carbon atom and the nod electron pairs around the carbon atom, but in nonclassical carbocations, the electrons are delocalized around the carbon atom. An example of a classical carbocation is methenium ion, while an example for a nonclassical carbocation is 2-norboryl ion.
Summary – Classical vs Nonclassical Carbocation
We can classify the carbocations into two groups as classical and noncalssical carbocations, depending on the chemical structure. The key difference between classical and nonclassical carbocation is that classical carbocations have a carbon atom having six electrons in three chemical bonds, whereas nonclassical carbocations have three-center two-electron structure. An example of a classical carbocation is methenium ion while an example of a nonclassical carbocation is 2-norboryl ion.
Reference:
1. “Carbocations.” Chemistry LibreTexts, 5 June 2019, Available here.
2. “Carbocation”. Wikipedia.Org, 2019, Available here.
Image Courtesy:
1. “The cyclopropyl carbinyl cation” By V8rik at English Wikipedia (CC BY-SA 3.0) via Commons Wikimedia
2. “Norbornyl Cation Single Well vs Double Well Potential” By Jcal730 – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vy5qqrKGTlrlurc2dZKennpi5or%2FSopqapF2YrrOuzpyYraGfo3w%3D