Difference Between Coefficient and Subscript
The key difference between coefficient and subscript is that coefficient gives the number of moles of a substance, whereas subscript gives the number of atoms present in a molecule.
The terms coefficient and subscript are very important in chemistry, specifically in writing chemical equations for reactions. Both these terms refer to numbers, but they give different details about a particular chemical reaction.
CONTENTS
1. Overview and Key Difference
2. What is Coefficient
3. What is Subscript
4. Side by Side Comparison – Coefficient vs Subscript in Tabular Form
5. Summary
What is Coefficient?
A coefficient is a number that gives the number of moles of a substance that takes part in a particular chemical reaction. We write this number in front of the substance when writing the chemical formula. Moreover, we can write the coefficient in the same size as other chemical symbols (not as a subscript or as a superscript). Let us consider the following chemical reaction as an example.
N2 + 3H2 ⟶ 2NH3
In the above chemical reaction, “3” in front of H2 and “2” in front of NH3 are coefficients. Although there is no coefficient in front of N2, you should know that there is “1”. These numbers express that this reaction requires one mole of nitrogen gas and three moles of hydrogen gas to completely react, giving two moles of ammonia.
What is Subscript?
A subscript is a number that gives the number of atoms present in a particular molecule. The number is important in writing the chemical formula of substances and chemical equations for reactions. Further, the subscript is written in a smaller size than other chemical symbols in the formula; we also write it at the bottom of the symbol of the particular atom. Let us consider the same example as above.
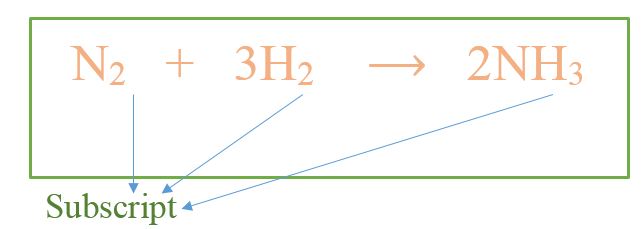
In the above example, nitrogen gas has two nitrogen atoms per molecule; thus, the subscript is “2”. For hydrogen gas, the subscript is the same. But in ammonia molecule, there is one nitrogen atom; thus, the subscript is “1”, but we don’t write it because as a general rule. Therefore, if it only has a symbol and no subscript, then it is essentially “1”. Ammonia molecule has three hydrogen atoms. Thus, the subscript there is “3”.
What is the Difference Between Coefficient and Subscript?
Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. The key difference between coefficient and subscript is that coefficient gives the number of moles of a substance, whereas subscript gives the number of atoms present in a molecule. For example, in the chemical equation “N2 + 3H2 ⟶ 2NH3”, the coefficient in front of nitrogen gas is 1, and the coefficient in front of hydrogen is 3; in front of ammonia, the coefficient is 2. For the same example, “N2 + 3H2 ⟶ 2NH3”, the subscript of nitrogen in nitrogen gas is 2, but in ammonia molecule, the subscript of nitrogen atom is 1.
Below infographic summarizes the difference between coefficient and subscript.
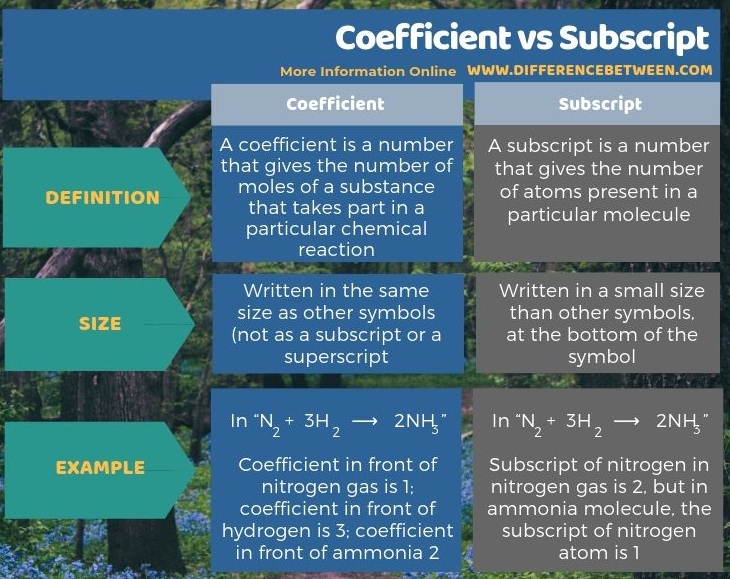
Summary – Coefficient vs Subscript
Both terms coefficient and subscript refer to numbers, but they give different details about the particular chemical reaction. The key difference between coefficient and subscript is that coefficient gives the number of moles of a substance, whereas subscript gives the number of atoms present in a molecule.
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vzp6dn6GTnrKvwIyapZ1lo6qvtK%2FRoqetZw%3D%3D