Difference Between Colorimetry and Spectrophotometry
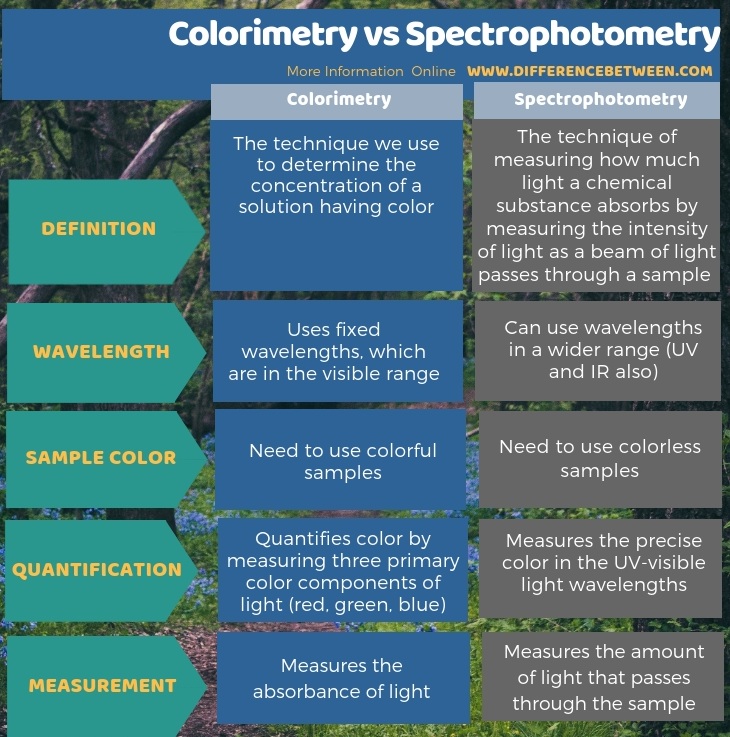
The key difference between colorimetry and spectrophotometry is that colorimetry uses fixed wavelengths that only are in the visible range while spectrophotometry can use wavelengths in a wider range.
Spectrophotometry and colorimetry are techniques we can use to identify molecules depending on their absorption and emission properties. Moreover, this is an easy technique to determine the concentration of a sample that has a color. Although molecules do not have a color, if we can make a colored compound from it by a chemical reaction, that compound can also be used in these techniques. Furthermore, energy levels are associated with a molecule, and they are discrete. Therefore, discrete transitions between the energy states will only occur at certain discrete energies. In these techniques, we measure the absorption and emission arising from these changes in the energy states. Thus, this is the basis of all spectroscopic techniques.
CONTENTS
1. Overview and Key Difference
2. What is Colorimetry
3. What is Spectrophotometry
4. Side by Side Comparison – Colorimetry vs Spectrophotometry in Tabular Form
5. Summary
What is Colorimetry?
Colorimetry is the technique that helps to determine the concentration of a solution having color. It measures the intensity of color and relates the intensity to the concentration of the sample. In colorimetry, the color of the sample is compared with a color of a standard in which the color is known.

Figure 1: Sampling in Colorimeter
Colorimeter is the equipment that we can use to measure the colored samples and give the appropriate absorptions.
What is Spectrophotometry?
Spectrophotometry is the technique of measuring how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. Moreover, the spectrophotometer is the instrument used in this technique. It has two main parts: the spectrometer, which produces the light with a selected color, and the photometer, which measures the intensity of light.

Figure 2: Spectrophotometer
In a spectrophotometer, there is a cuvette where we can place our liquid sample. The liquid sample will have a color, and it absorbs its complementary color when a light beam passes through it. The color intensity of the sample relates to the concentration of the substance in the sample. Therefore, that concentration can be determined by the extent of absorption of light at the given wavelength.
What is the Difference Between Colorimetry and Spectrophotometry?
Both colorimetry and spectrophotometry are quantitative measurements for the determination of the amount of substance present in a sample. The key difference between colorimetry and spectrophotometry is that the colorimetry uses fixed wavelengths that are only in the visible range while spectrophotometry can use wavelengths in a wider range.
Moreover, a significant difference between colorimetry and spectrophotometry is that a colorimeter quantifies color by measuring three primary color components of light (red, green, blue), whereas a spectrophotometer measures the precise color in human-visible light wavelengths. Furthermore, colorimeter measures the absorbance of light whereas spectrophotometer measures the amount of light that passes through the sample. So, this is also a difference between colorimetry and spectrophotometry.
Summary – Colorimetry vs Spectrophotometry
In brief, colorimetry and spectrophotometry are two methods we can use to determine the content of a substance in a given sample by measuring light absorption through that sample. The key difference between colorimetry and spectrophotometry is that colorimetry uses wavelengths that are only in the visible range while spectrophotometry can use wavelengths in a wider range.
Reference:
1. RITA CORNELIS, MONICA NORDBERG, in Handbook on the Toxicology of Metals (Third Edition), 2007
2. “Spectrophotometry.” Chemistry LibreTexts, Libretexts, 21 Apr. 2019, Available here.
Image Courtesy:
1. “Chlorine colorimeter” By U.S. Air Force photo/Senior Airman Chase Hedrick – (Public Domain) via Commons Wikimedia
2. “Spectrophotometer Model 2” By Viv Rolfe – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26vzqWmq6GdmsGzxYyapZ1lpqh6tLzEnKurp6CdvLW7zJ6rq7Ff