Difference Between Cyclobutane and Cyclopropane
The key difference between cyclobutane and cyclopropane is that cyclobutane is a cyclic structure having four carbon atoms in a ring structure whereas cyclopropane is a cyclic structure having three carbon atoms in a ring structure.
Cyclobutane and cyclopropane are two organic compounds having ring structures with carbon atoms arranged in a cycle. The difference between cyclobutane and cyclopropane depends on the number of carbon atoms in the ring.
CONTENTS
1. Overview and Key Difference
2. What is Cyclobutane
3. What is Cyclopropane
4. Side by Side Comparison – Cyclobutane vs Cyclopropane in Tabular Form
5. Summary
What is Cyclobutane?
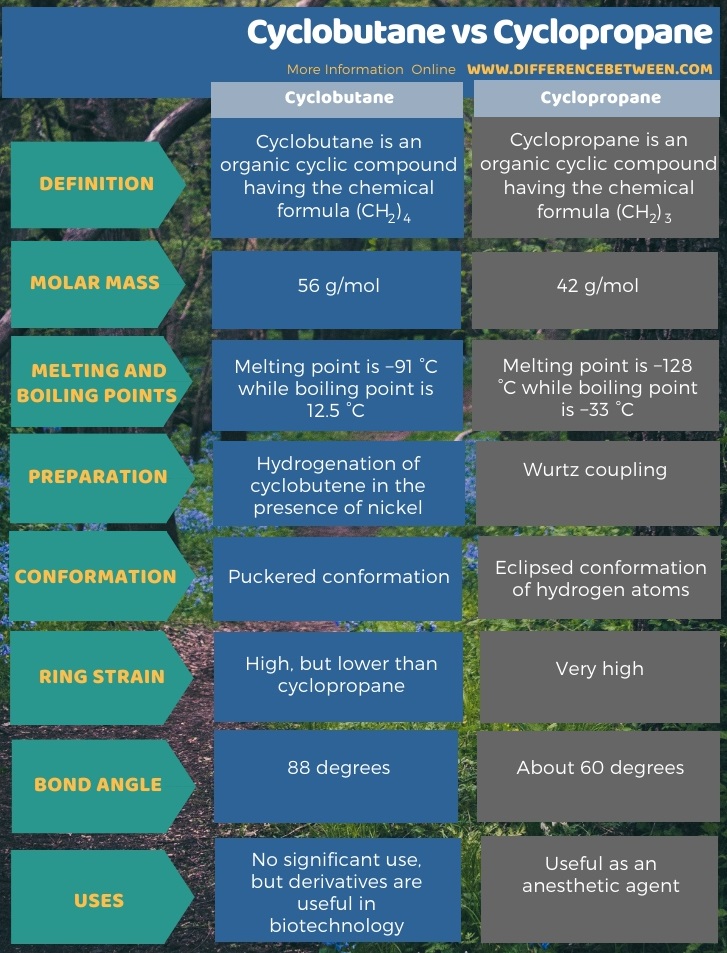
Cyclobutane is an organic cyclic compound which has the chemical formula (CH2)4. It exists as a colourless gas, which is commercially available as a liquefied gas. The molar mass of this compound is 56 g/mol. The melting point of this compound is −91 °C while the boiling point 12.5 °C. When considering the bond angles of this compound, there is a significant strain between the carbon atoms. Due to this ring strain, the cyclobutane structure has lower bond energy compared to its linear structure or unstrained structure. However, the cyclobutane molecule is unstable at temperatures above 500 °C.
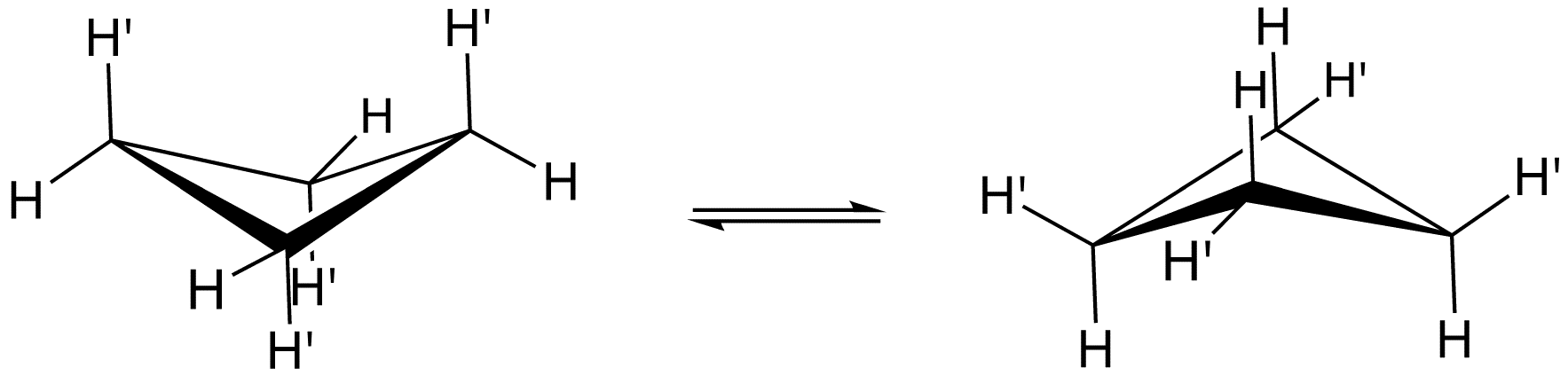
Figure 01: Interconversion of the Puckered Structure
There are four carbon atoms in this cyclic structure; usually, these four carbon atoms do not form a coplanar structure. It exists as a folded, “puckered” conformation. In this conformation, some eclipsed interactions are reduced. There are many different ways of preparing cyclobutene, but the earliest and the most efficient method is hydrogenation of cyclobutene in the presence of nickel as the catalyst.
What is Cyclopropane?
Cyclopropane is an organic cyclic compound which has the chemical formula (CH2)3. It contains three carbon atoms linked to each other, forming a ring structure, and each carbon atoms in this ring bear two hydrogen atoms. The molecular symmetry of this molecule can be defined as D3h symmetry. Furthermore, there is a high ring strain due to the small ring structure.
Cyclopropane occurs as a colourless gas which has a sweet odour. The molar mass of this compound is 42 g/mol. The melting point of this compound is −128 °C while the boiling point is −33 °C. Moreover, cyclopropane can act as an anaesthetic when inhaled.
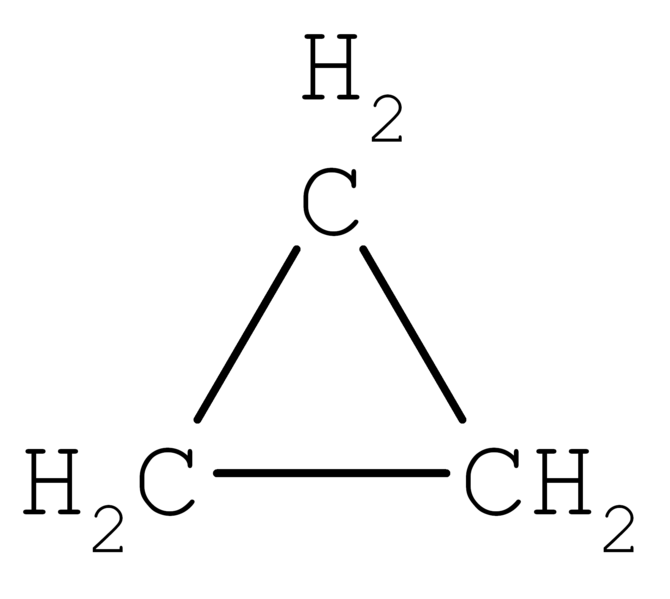
Figure 02: A Cyclopropane
Apart from the ring strain, which arises due to the reduced bond angles, there is also torsional strain because of the eclipsed conformation. Therefore, the chemical bonds in this structure are comparatively weaker than the corresponding alkane. The earliest method of production of cyclopropane was from Wurtz coupling.
What is the Difference Between Cyclobutane and Cyclopropane?
Cyclobutane and cyclopropane are organic compounds which have carbon atoms arranged in a cycle. The key difference between cyclobutane and cyclopropane is that cyclobutane is a cyclic structure having four carbon atoms in a ring structure whereas cyclopropane is a cyclic structure having three carbon atoms in a ring structure.
Moreover, both these structures show ring strain due to the reduced bond angles, but the ring strain in cyclopropane is much higher than that of cyclobutane due to the lower bond angle. Besides, there is a torsional strain in cyclopropane due to the eclipsed conformation of the hydrogen atoms. So, this is another difference between cyclobutane and cyclopropane. When considering the preparation method, the earliest and the most efficient method of the production of cyclobutane is hydrogenation of cyclobutene in the presence of nickel as the catalyst, while the earliest method of production of cyclopropane was from Wurtz coupling.
Below infographic shows more comparisons on the difference between cyclobutane and cyclopropane.
Summary – Cyclobutane vs Cyclopropane
Cyclobutane and cyclopropane are organic compounds having ring structures with carbon atoms arranged in a cycle. The key difference between cyclobutane and cyclopropane is that cyclobutane is a cyclic structure having four carbon atoms in a ring structure whereas cyclopropane is a cyclic structure having three carbon atoms in a ring structure.
Reference:
1. “Cyclopropane | Chemical Compound”. Encyclopedia Britannica, 2019, Available here. Accessed 6 Dec 2019.
Image Courtesy:
1. “CyclobutaneConf2” By Smokefoot – Own work (Public Domain) via Commons Wikimedia
2. “Cyclopropane” (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26v2JyjqJqlqa6vsYyapZ1lk66wrbvPq6apmZ6afA%3D%3D