Difference Between Distillation and Condensation
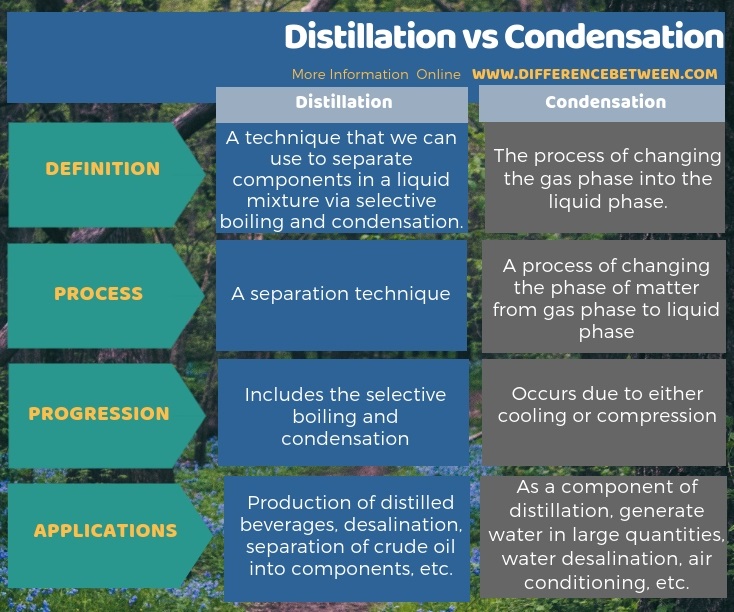
The key difference between distillation and condensation is that the distillation is a separation technique whereas the condensation is a process of changing the phase of matter.
Distillation is a common technique in industries that we can use to separate components in a liquid mixture. The technique includes selective boiling followed by condensation. On the other hand, condensation is the process of changing the phase of matter from gas phase to liquid phase.
CONTENTS
1. Overview and Key Difference
2. What is Distillation
3. What is Condensation
4. Side by Side Comparison – Distillation vs Condensation in Tabular Form
5. Summary
What is Distillation?
Distillation is a technique that we can use to separate components in a liquid mixture via selective boiling and condensation. Most of the times, this technique gives a complete separation. However, sometimes, it may result in a partial separation. Here, the complete separation gives nearly pure compounds while the partial separation gives an increased concentration of some selected components in the liquid mixture.

Figure 01: An Apparatus for Fractional Distillation
Furthermore, this process depends mainly on the volatility of the components in the mixture. Also, this technique involves a physical separation rather than involving chemical reactions. Besides, there are several distillation techniques in laboratory scale which includes, simple distillation, fractional distillation, steam distillation, vacuum distillation, etc.
Applications:
- To produce distilled beverages with a high alcoholic content
- For desalination
- Partial distillation of crude oil for safe storage and transport
- Cryogenic distillation for the separation of normal air into gaseous components
- To separate components in crude oil
What is Condensation?
Condensation is the process of changing the gas phase into the liquid phase. In contrast, vaporization is the change of the liquid phase into the gas phase. Thus, this term often refers to the condensation of water; change of water vapour into liquid water. Above all, the condensation occurs when we cool or compress a vapour to its saturation limit until the molecular density in the gas phase reaches its maximum capacity. The equipment that we can use for this cooling and compressing are “condensers”.

Figure 02: Condensation on the Water Bottle
When considering the process, it initiates with the formation of molecular clusters within the gaseous volume. However, sometimes it initiates with the contact of the gaseous phase with a liquid surface. For instance, rain drops or snowflakes form within clouds. There, it is catalyzed by water-nucleating proteins. Here, the atmospheric microbes produce these proteins.
Applications:
- As a component of a distillation
- To generate water in large quantities for human use
- Power generation
- Water desalination
- Refrigeration
- Air conditioning
What is the Difference Between Distillation and Condensation?
Distillation is a technique that we can use to separate components in a liquid mixture via selective boiling and condensation while condensation is the process of changing the gas phase into liquid phase. Therefore, the key difference between distillation and condensation is that distillation is a separation technique whereas condensation is a process of changing the phase of matter. A further difference between distillation and condensation is that the distillation uses the differences in boiling points of components in the liquid mixture while the condensation occurs due to cooling or compression of a vapour.
Moreover, there is a difference between distillation and condensation based on applications too. That is; the applications of distillation include the production of distilled beverages, desalination, separation of crude oil into components, etc. Whereas, the applications of condensation include using it as a component of distillation, to generate water in large quantities, water desalination, air conditioning, etc.
Summary – Distillation vs Condensation
Distillation is very important in different industries. Mainly, it is an important process in crude oil refining. On the other hand, condensation is also an important process, but we use it mainly regarding the condensation of water vapour. However, condensation is an essential component in distillation too. In summary, the key difference between distillation and condensation is that the distillation is a separation technique whereas the condensation is a process of changing the phase of matter.
Reference:
1. “Distillation.” Wikipedia, Wikimedia Foundation, 6 Nov. 2018. Available here
2. “Condensation.” Wikipedia, Wikimedia Foundation, 26 Oct. 2018. Available here
Image Courtesy:
1.”Double Distilled Water Unit”By Guruleninn – Own work, (CC BY-SA 3.0) via Commons Wikimedia
2.”Condensation on water bottle” (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26wyKyroqSclsGqu81mmKecXZi8r7DEp6qarJmku3A%3D