Difference Between Electronic Rotational and Vibrational Transition
The key difference between electronic rotational and vibrational transition is that electronic transitions occur between different electronic states while rotational transitions occur in the same vibrational state and vibrational transitions occur in the same electronic state.
Electronic, rotational and vibrational transitions can be described as properties of molecules. We can investigate molecular structure as a parallel study to the atomic structure using the methods of quantum mechanics and the information obtained from molecular spectra. The most common molecular spectra include electronic, rotational and vibrational transitions.
CONTENTS
1. Overview and Key Difference
2. What is Electronic Transition
3. What is Rotational Transition
4. What is Vibrational Transition
5. Side by Side Comparison – Electronic vs Rotational vs Vibrational Transition in Tabular Form
6. Summary
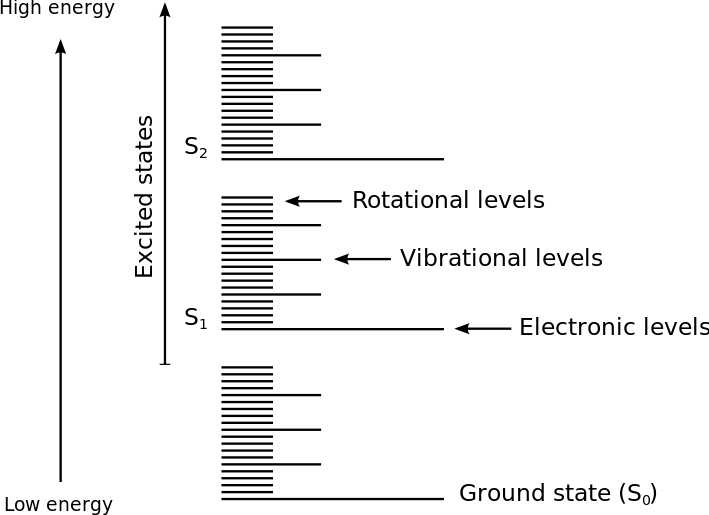
What is Electronic Transition?
Electronic transitions in molecules take place when electrons in the molecule become excited from one energy level to another. Here, the electrons tend to move from a low energy level to a high energy level. The change in the energy that is associated with this transition provides information about the structure of the molecule and helps in determining the molecular properties such as colour. The relationship between the energy and the frequency of radiation that are used in the transition process can be given by the Planck’s relation.
In organic compounds, we can easily determine the electronic transitions via UV-visible spectroscopy. Here, the transitions of the molecule should exist in the UV and visible range of the electromagnetic spectrum. Usually, the electrons in a HOMO of a sigma bond get excited to the LUMO of the same bond. Likewise, an electron in the pi bonding orbital can get excited to the antibonding pi orbital. However, the electronic transitions of molecules strongly depend on the type of solvent that is used in the analysis.
What is Rotational Transition?
Rotational transitions of molecules refer to the abrupt change in the angular momentum of that molecule. This definition is given depending on the theories of quantum physics, which states that angular momentum of a molecule is a quantized property and it can only equal certain discrete values that correspond to different rotational energy states. The rotation transition refers to the loss or gain of angular momentum, which causes the molecule to move either to a higher or a lower rotational energy state.
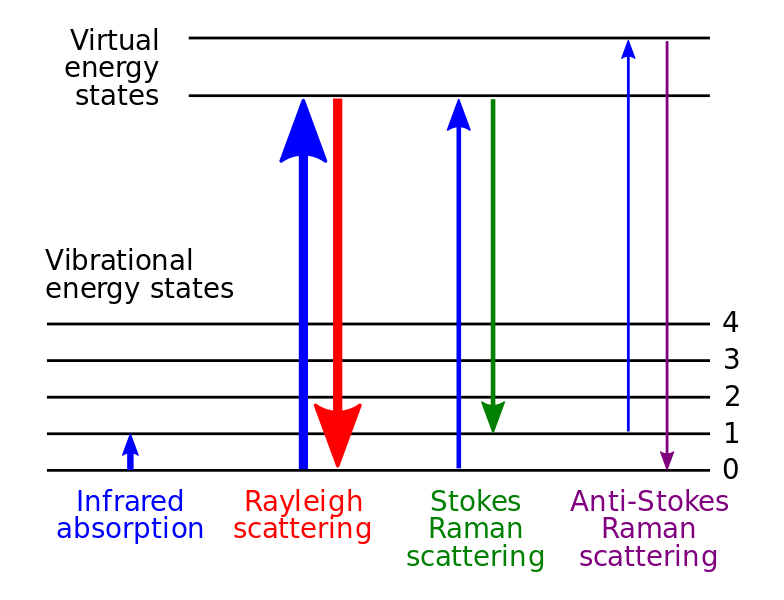
Rotational transitions create unique spectral lines in a spectrum. When there is a net gain or loss of energy during a transition, the molecule should either absorb or emit a particular frequency of the EMR or electromagnetic radiation. This process forms discrete spectral lines, and we can easily detect these lines via a spectrometer through either rotational spectroscopy or Raman spectroscopy.
What is Vibrational Transition?
Vibrational transition of a molecule refers to the movement of the molecule from one vibrational energy level to another. We can also name it as vibronic transition. This type of transition occurs in between different vibrational levels of the same electronic state.
In order to evaluate the vibrational transition of a particular molecule, we should know the dependence of the molecule-fixed components of the electric dipole moment on the molecular deformations. Generally, Raman spectroscopy is based on vibrational transitions.
What is the Difference Between Electronic Rotational and Vibrational Transition?
Electronic, rotational and vibrational transitions are important in the determination of molecular structure using molecular spectra. The key difference between electronic rotational and vibrational transition is that electronic transitions occur between different electronic states while rotational transitions occur in the same vibrational state and vibrational transitions occur in the same electronic state.
Below is a summary of the difference between electronic rotational and vibrational transition.
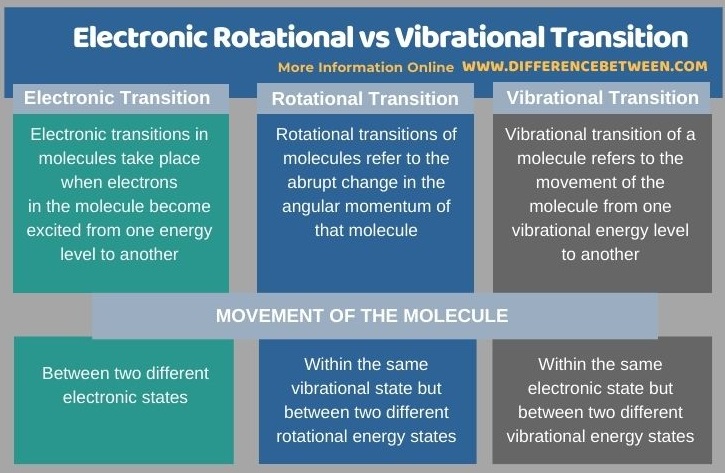
Summary – Electronic Rotational vs Vibrational Transition
Electronic, rotational and vibrational transitions are important in the determination of molecular structure using molecular spectra. The key difference between electronic rotational and vibrational transition is that electronic transitions occur between different electronic states while rotational transitions occur in the same vibrational state and vibrational transitions occur in the same electronic state.
Reference:
1. Nave, R. “Properties of Molecules.” HyperPhysics, Georgia State University, Available here.
2. “15.2: Vibration-Rotation Transitions.” Chemistry LibreTexts, Libretexts, 14 July 2020, Available here.
Image Courtesy:
1. “Raman energy levels” By Moxfyre, based on work of User:Pavlina2.0 – vectorization of File:Raman energy levels.jpg (CC BY-SA 3.0) via Commons Wikimedia
2. “Molecular energy levels en” By Lvzon – Own work by uploader, based loosely on figure 1a in Lichtman & Cochello, 1995 (DOI: 10.1038/nmeth817) (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26xy56araqfo7akedGoq5qsmaS7oriMmqWdZaaer7Ot06Kmp5mcYsGzrc2soK2hn6N8