Difference Between FeO and Fe2O3
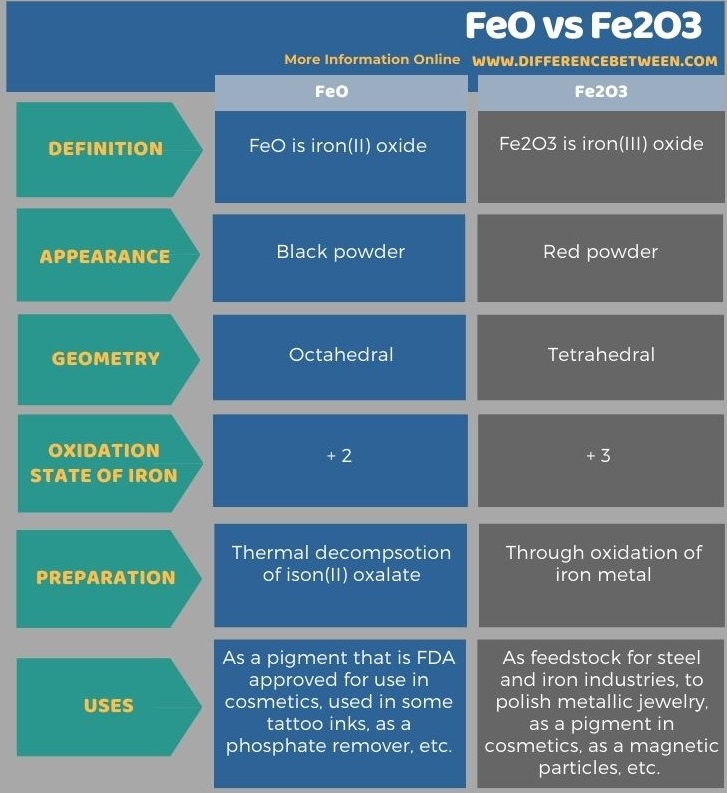
The key difference between FeO and Fe2O3 is that FeO has iron in +2 oxidations state, whereas Fe2O3 has iron in +3 oxidation state.
In brief, FeO and Fe2O3 are oxides of iron but having the iron atoms in different oxidation states. FeO is the chemical formula of iron(II) oxide while Fe2O3 is the chemical formula of iron(III) oxide. These two substances have different chemical properties, appearances, as well as different uses. In this article, we will be discussing these properties and comparing them to discern the difference between FeO and Fe2O3.
CONTENTS
1. Overview and Key Difference
2. What is FeO
3. What is Fe2O3
4. Side by Side Comparison – FeO vs Fe2O3 in Tabular Form
5. Summary
What is FeO?
FeO is iron(II) oxide. It is also known as ferrous oxide. It is an inorganic compound that appears as black coloured crystals. Due to its appearance, sometimes people confuse it with rust. The mineral form of this substance is wustite. This compound has a molar mass of 71.84 g/mol and is insoluble in water. Moreover, FeO is insoluble in alkali alcohol, but it dissolves in acids.
We can produce FeO through the thermal decomposition of iron(II) oxalate. We can conduct this procedure under an inert atmosphere in order to avoid the formation of iron(II) oxide.

Figure 01: FeO
Thermodynamically, FeO is unstable at low temperatures (below 575 Celsius degrees); therefore, it tends to disproportionate into metal and Fe3O4, another common oxide of iron. Generally, iron(II) oxide has a cubic, rock salt structure in which iron atoms are arranged in an octahedral manner that is coordinated by oxygen atoms. All oxygen atoms are also coordinated by iron atoms octahedrally. However, we say this substance is non-stiochiometric because some of the Fe(II) ions in this substance are easily replaced by Fe(III) ions, and these Fe(III) ions have a tetrahedral geometry around them, unlike the octahedral geometry of Fe(II) ions.
Approximately, 9% of the Earth’s mantle is made of FeO, and within the mantle, this material might occur as an electrically conductive material which possibly explains the perturbations in Earth’s rotation.
When considering the uses of FeO, it is useful as a pigment that is FDA approved for use in cosmetics. We can also use it in some tattoo inks. Moreover, this substance is important as a phosphate remover from home aquaria.
What is Fe2O3?
Fe2O3 is iron(III) oxide. The red iron oxide is ferric oxide and has the chemical formula Fe2O3. Its chemical name is iron(III) oxide. Moreover, it is a major oxide of iron, and in mineralogy, we call this compound “hematite”. It is the main source of iron for the steel industry and is ferromagnetic. Its molar mass is 159.69 g/mol while its melting point is around 1,539–1,565 °C. It easily decomposes at higher temperatures. Therefore, this compound is insoluble in water.

Figure 02: Fe2O3
Furthermore, there are different structures of this compound; we call them “polymorphs”. Ex: alpha phase, gamma phase, etc. In every structure, one iron cation binds with six oxygen ligands (around the iron cation). Moreover, there are some hydrated forms of this compound as well. More importantly, red iron oxide occurs as a red-brown solid. Hence, it is a good indicator for us to recognize this compound from other iron oxides.
What is the Difference Between FeO and Fe2O3?
FeO and Fe2O3 are oxides of iron having different oxidation states of iron atoms. The key difference between FeO and Fe2O3 is that FeO has iron in +2 oxidations state, whereas Fe2O3 has iron in +3 oxidation state. Moreover, FeO is a black powder while Fe2O3 is a red powder.
The following infographic tabulates more differences between FeO and Fe2O3.
Summary – FeO vs Fe2O3
FeO and Fe2O3 are oxides of iron having different oxidation states of iron atoms. They also have different appearances as well. The key difference between FeO and Fe2O3 is that FeO has iron in +2 oxidations state, whereas Fe2O3 has iron in +3 oxidation state.
Reference:
“Iron(III) Oxide – Structure, Properties, Uses of Fe2O3. ” Byjus.com, Available here.
Image Courtesy:
1. “Iron(II) oxide” (Public Domain) via Commons Wikimedia
2. “Iron(III)-oxide-sample” By Benjah-bmm27 – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26yxKhkmqaUYrOmfs5sZg%3D%3D