Difference Between Gram Equivalent and Equivalent Weight
The key difference between gram equivalent and equivalent weight is that gram equivalent describes that the mass of one equivalent is given in the unit of gram whereas equivalent weight describes the mass of one equivalent in any unit of measurement.
Gram equivalent and equivalent weight are two terms that are used in general chemistry for chemical calculations. These terms also have uses in analytical chemistry (in volumetric and gravimetric analysis) and in polymer chemistry.
CONTENTS
1. Overview and Key Difference
2. What is Gram Equivalent
3. What is Equivalent Weight
4. Side by Side Comparison – Gram Equivalent vs Equivalent Weight Reaction in Tabular Form
5. Summary
What is Gram Equivalent?
Gram equivalent is the mass of an equivalent in the unit of grams. It describes the mass in grams of an element, group, or a compound. However, this term is different from the term equivalent weight based on the unit of measurement. This is because the mass can be measured in different units and an equivalent means any considered part of a material. Then the mass of that part is expressed in the unit of measurement for the mass of that particular material.
What is Equivalent Weight?
Equivalent weight refers to the mass of one equivalent of a material that is considered. It can be used with an element, a group of elements or a compound. Also, this term can be defined as the mass of a known substance that can combine or displace a fixed quantity of another substance. For example, the equivalent weight of an element is the mass which combines with or displaces 1.008 grams of hydrogen, 8.0 grams of oxygen or 35.5 grams of chlorine. These values are obtained by dividing the atomic mass from the most common valence value; e.g. equivalent weight with regard to oxygen is obtained by 16gmol-1/2=8.0g.
However, for acid-base reactions, the equivalent weight refers to the mass that reacts with a mole of hydrogen ions. The unit of equivalent weight is the unit of mass. Therefore, this is not a dimensionless value. Most commonly, gram is the unit used to measure the equivalent weight. Originally, the value of the equivalent weight is determined experimentally. But, we can calculate it using the molar masses as well. For example, the equivalent weight can be calculated by dividing the molar mass from positive or negative electrical charges formed from the dissolution of the compound.
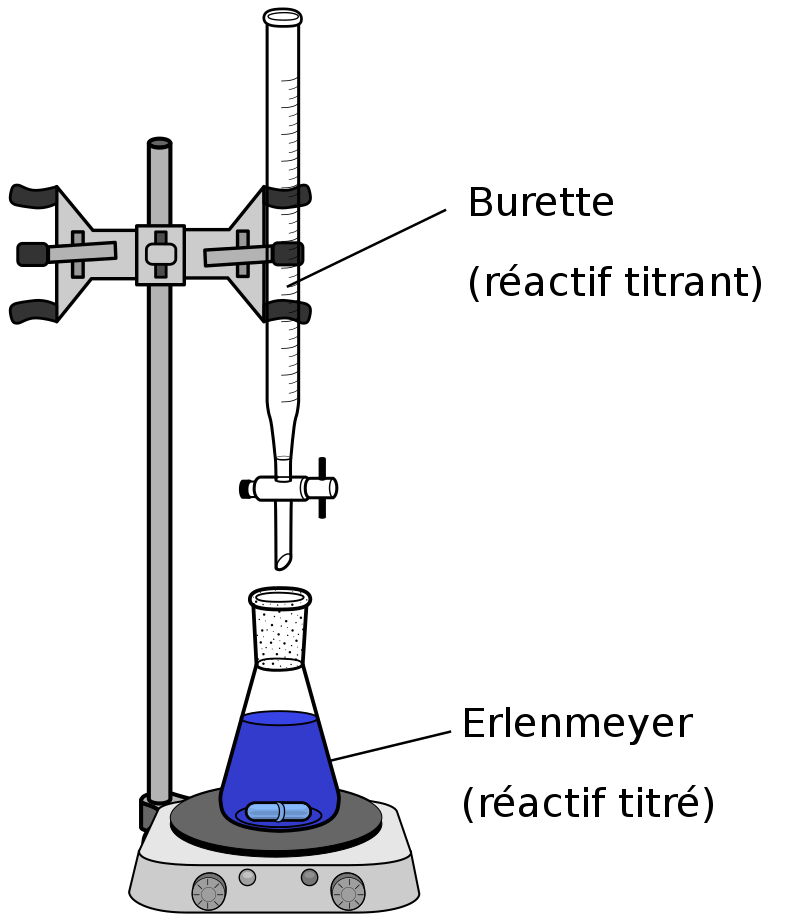
Figure 1: An Acid-Base titration (the calculations in these titration techniques use the equivalent weights of acids and bases to avoid difficulty in calculations)
In general chemical calculations, we can determine the equivalent weight by dividing the molar mass of the desired substance from the number of moles of hydrogen ions produced by this compound upon dissolution in water. For example, the molar mass of sulfuric acid is 98 g/mol. It produces two moles of hydrogen ions upon dissolution. Therefore, the equivalent weight is 98/2=49geq-1.
What is the Difference Between Gram Equivalent and Equivalent Weight?
Gram equivalent and equivalent weight are important terms we use in chemical calculations. The key difference between gram equivalent and equivalent weight is that gram equivalent describes that the mass of one equivalent is given in the unit of gram whereas equivalent weight describes the mass of one equivalent in any unit of measurement.
The below infographic summarizes the difference between gram equivalent and equivalent weight.
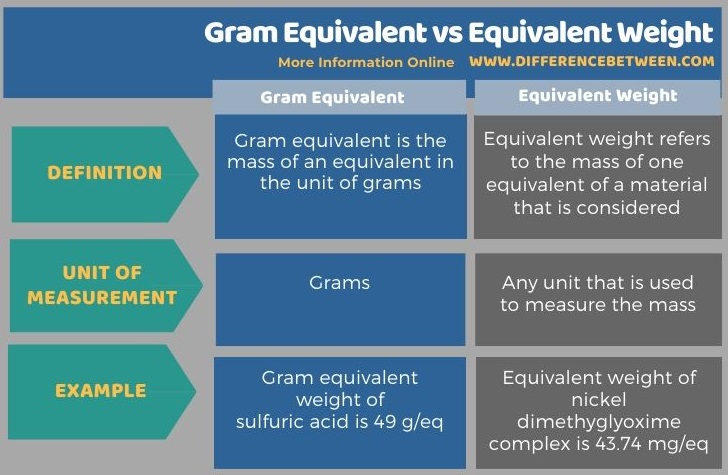
Summary – Gram Equivalent vs Equivalent Weight
Gram equivalent and equivalent weight are important terms that we use in chemical calculations. The key difference between gram equivalent and equivalent weight is that gram equivalent describes that the mass of one equivalent is given in the unit of gram whereas equivalent weight describes the mass of one equivalent in any unit of measurement.
Reference:
1. “Equivalent Weight.” OpenStax CNX, Available here.
2. “Equivalent Weight.” Wikipedia, Wikimedia Foundation, 29 Dec. 2019, Available here.
Image Courtesy:
1. “Acid-base-titration-fr” By Mehinger – Syrabas-titrering.svg (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26z0ZqkZp2hqra3rcuepa1lkaOxbrHQrqCvmZyau7V51p6goKCkZA%3D%3D