Difference Between Hexagon and Monoclinic Unit Cell
The key difference between hexagon and monoclinic unit cell is that hexagon unit cell has two axes with equal length and one axis with a different length whereas monoclinic unit cell has all three axes with unequal lengths.
A unit cell is the basic unit of a crystal system that represents the repeating pattern of the crystal system. And this unit cell is a box-like structure. Therefore, it resembles all the atoms with their spatial arrangement. Moreover, this box has three axes (a, b and c) and three angles (α, β and γ). These axes and angles are useful in defining the type of unit cell.
CONTENTS
1. Overview and Key Difference
2. What is Hexagon Unit Cell
3. What is Monoclinic Unit Cell
4. Side by Side Comparison – Hexagon vs Monoclinic Unit Cell in Tabular Form
5. Summary
What is Hexagon Unit Cell
Hexagon unit cell or the hexagonal unit cell is the basic unit that represents all the atoms and their arrangement in a hexagonal crystal system. This hexagon unit cell has two axes with equal lengths, and the remaining axis has a different length from those two axes.
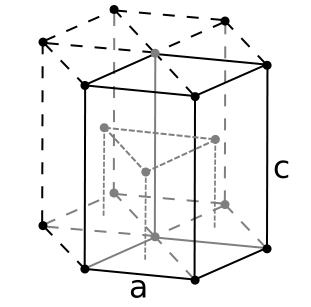
Figure 01: Hexagonal Unit Cell
This axis with a different length is perpendicular to the other two axes. That is, a=b≠c. When considering the angles between these axes, the angle between a and b axes (axes with equal lengths) is 120◦ while other two angles equal to 90◦.
What is Monoclinic Unit Cell?
The monoclinic unit cell is the basic unit that represents all the atoms and their arrangement in a monoclinic crystal system. Therefore, in this unit cell, all the three axes have unequal lengths. That is, a≠b≠c. Furthermore, this type of unit cells has a rectangular shape.
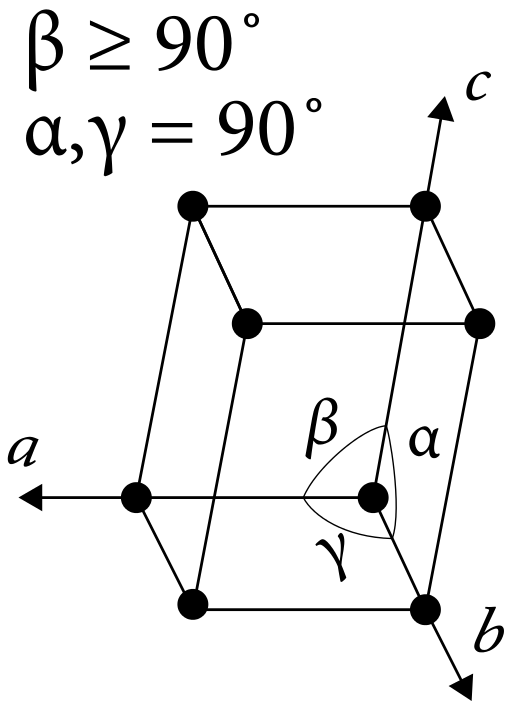
Figure 02: Monoclinic Unit Cell
The base of this unit cell is a parallelogram (which has two pairs of parallel sides). The angles of this unit cell are α, γ, β where α=γ=90◦ and β≠90◦.
What is the Difference Between Hexagon and Monoclinic Unit Cell?
Hexagon vs Monoclinic Unit Cell | |
| The hexagon unit cell or the hexagonal unit cell is the basic unit that represents all the atoms and their arrangement in a hexagonal crystal system. | The monoclinic unit cell is the basic unit that represents all the atoms and their arrangement in a monoclinic crystal system. |
| Three Axes | |
| Hexagon unit cell has two axes with equal lengths, and the remaining axis has a different length from those two axes (a=b≠c). | The monoclinic unit cell has its three axes with unequal lengths (a≠b≠c). |
| Angles | |
| It has α and β angles equal to 90° and γ equal to 120°. | It has α and γ angles equal to 90°, and β is not equal to 90°. |
| Parallelogram | |
| There are no Parallelograms in a hexagonal unit cell. | The base of the monoclinic unit cell is a Parallelogram. |
Summary – Hexagon vs Monoclinic Unit Cell
The difference between hexagon and monoclinic unit cell is that hexagon unit cell has two axes with equal length and one axis with a different length whereas monoclinic unit cell has all three axes with unequal lengths.
Reference:
1. “Hexagonal Crystal System | Earth Sciences Museum.” Campus Wellness, 9 Oct. 2013. Available here
2. “Monoclinic Crystal System.” Wikipedia, Wikimedia Foundation, 10 Apr. 2018. Available here
Image Courtesy:
1.’Hexagonal close packed’By User:Dornelf (CC BY-SA 3.0) via Commons Wikimedia
2.’Monoclinic cell’By Mahleritederivative work (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau260xLGYoKeeYq6vsIympqenk6G2r7XCZqynoaRisKa4y2g%3D