Difference Between Hydroxyl and Hydroxide
Key Difference – Hydroxyl vs Hydroxide
The two terms hydroxyl and hydroxide sound very similar since both of them have two similar atoms, Oxygen (O=16) and Hydrogen (H=1). Hydroxide is a negative ion with a single charge and hydroxyl is not found in its free form, it is a part of another molecule or ion. Hydroxide ions are more reactive than the hydroxyl group in a molecule. This is the key difference between hydroxyl and hydroxide.
What is Hydroxyl?
Hydroxyl is a neutral compound and it is the corresponding electrically neutral compound of hydroxide ion. The free form of hydroxyl (•HO) is a radical and when it is bonded covalently to other molecules it is denoted as the hydroxyl (–OH) group. Hydroxyl groups can act as nucleophiles and hydroxyl radical is used as a catalyst in organic chemistry. Hydroxyl groups are not highly reactive as the other nucleophiles. However, they are the facilitators in the formation of strong intramolecular forces called ‘hydrogen bonds’.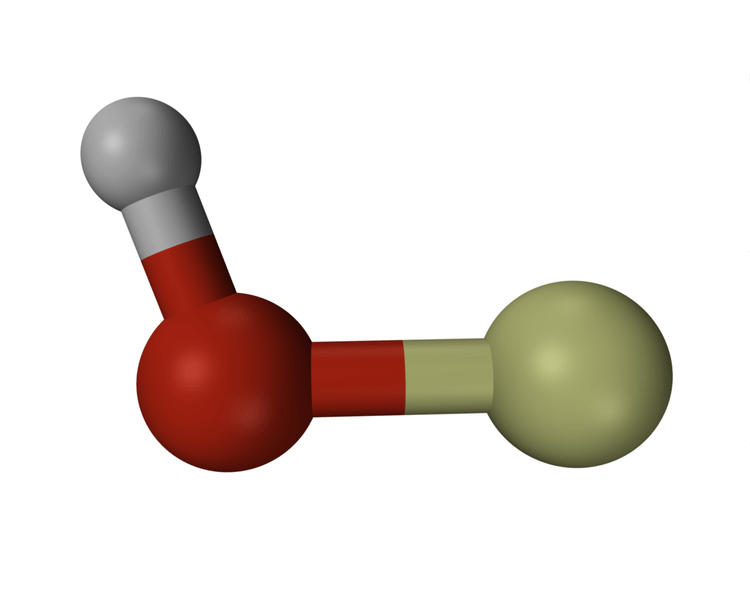
What is Hydroxide?
Hydroxide is a diatomic anion which contains an oxygen atom and a hydrogen atom. The bond between oxygen and hydrogen atom is covalent and its chemical formula is OH–. Self-ionization of water produces hydroxyl ions and therefore hydroxyl ions are a natural part in the water. Hydroxide ions are utilized as a base, a ligand, a nucleophile and a catalyst in chemical reactions. In addition, hydrogen ions produce salts with metal cations and most of them dissociate in aqueous solutions, releasing solvated hydroxide ions. Many inorganic chemical substances contain the term “hydroxide” in their name, but they are not ionic and they are covalent compounds which contain hydroxyl groups.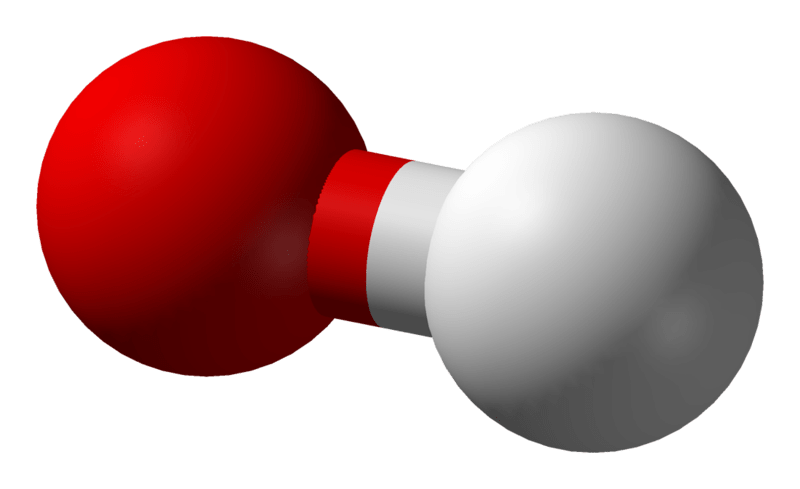
What is the difference between Hydroxyl and Hydroxide?
Structure:
Hydroxyl: Hydroxyl is an electrically neutral compound which can be found two ways, as the radical and the covalently bound form.

Hydroxyl radical When it is covalently bonded to a molecule
Hydroxide: Hydroxide is a negatively charged ion and the negative charge is on the oxygen atom.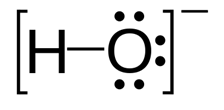 Properties:
Properties:
Hydroxyl: Hydroxyl groups are found in many organic compounds; alcohols, carboxylic acids and hydroxyl groups containing sugar. Compounds containing hydroxyl groups such as water, alcohols, and carboxylic acids can be easily deprotonated. In addition, these hydroxyls groups are engaged in the formation of hydrogen bonds. Hydrogen bonds help molecules to stick together and this leads to the possession of higher boiling and melting points. In general, organic compounds are poorly water soluble; these molecules become slightly water soluble when they contain two or more hydroxyl groups.
Hydroxide: Most of the chemicals which contain hydroxide are considered as very corrosive, and some are very harmful.When these chemicals are dissolved in water, the hydroxide ion acts as an incredibly strong base. Since hydroxide ion bears a negative charge, it is often bonded to positively charged ions.
Some ionic compounds containing hydroxide groups in their molecule dissolve really well in water; corrosive bases such as sodium hydroxide (NaOH) and potassium hydroxide (KOH) can be taken as examples. However, some other hydroxide containing ionic compounds are slightly insoluble in water; examples are copper (II) hydroxide [Cu(OH)2 – bright blue coloured] and iron (II) hydroxide[Fe(OH)2– brown].
Reactivity:
Hydroxyl: Hydroxyl groups are less reactive compared to the hydroxide group. But, hydroxyl groups readily form hydrogen bonds and contribute to making molecules more soluble in water.
However, hydroxyl radicals are highly reactive and very useful in organic chemical reactions.
Hydroxide: Hydroxide (OH–) group is considered as a strong nucleophile in Organic chemistry.
References: [email protected], M. C. (n.d.). Hydroxide ions. Retrieved December 28, 2016, from here Hydroxide Ion: Definition & Formula – Video & Lesson Transcript. (n.d.). Retrieved December 28, 2016, from here Hydroxide. (n.d.). Retrieved December 28, 2016, from here Hydroxy group. (n.d.). Retrieved December 28, 2016, from here Hydroxyl Group: Definition, Structure & Formula. (n.d.). Retrieved December 28, 2016, from here H. (2014). What Is a Hydroxyl Group? Retrieved December 28, 2016, from herencG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2602J2pqLCpoXqiusNmraxlmK6xs7vXopueZw%3D%3D