Difference Between Instantaneous Rate and Average Rate
Key Difference – Instantaneous Rate vs Average Rate
In chemical reactions, the reaction rate can be determined in two ways as instantaneous rate and average rate. The key difference between instantaneous rate and average rate is that instantaneous rate measures the change in concentration of reactants or products during a known time period whereas average rate measures the change in concentration of reactants or products during the whole time taken for the completion of the chemical reaction.
The reaction rate of a chemical reaction is the measure of change in the concentration of reactants used up or the products formed during the reaction. It is also known as the speed of reaction.
CONTENTS
1. Overview and Key Difference
2. What is Instantaneous Rate
3. What is Average Rate
4. Side by Side Comparison – Instantaneous Rate vs Average Rate in Tabular Form
5. Summary
What is Instantaneous Rate?
Instantaneous rate is the rate of a chemical reaction that is measured as the change of the concentration of reactants or the products during a known time period. In this method, the rate of the reaction during a specific instant in time is measured. It can also be measured as the rate of the reaction at a particular moment. The instantaneous rate is also known as differential rate.
The reaction rate of a chemical reaction is often different from one point to another during the progression of the reaction (reaction rate changes continuously). The reaction rate slows when reactants are used up for the reaction. This is because the concentration of reactants decreases with the progression of the reaction (reactants are consumed by the chemical reaction).
Instantaneous Rate of Change Formula
The instantaneous rate is given as below.
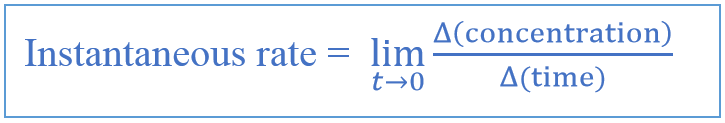
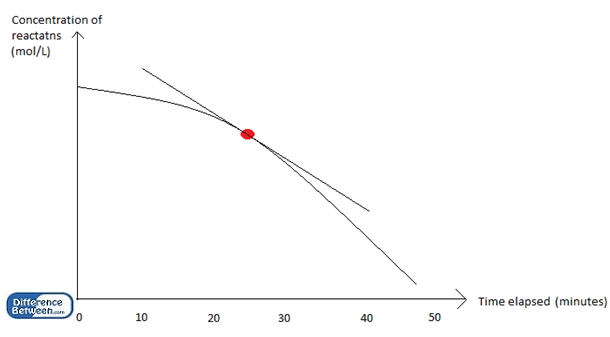
Above graph shows the decrease of concentration of reactants with time in a chemical reaction; the instantaneous rate is the rate of the reaction at a particular point (red colored point); the average rate is calculated by dividing the total concentration of reactants (at the beginning) from the total time (50 minutes).
What is Average Rate?
Average rate is the rate of a chemical reaction that is measured as the change of the concentration of reactants or the products during the whole time period of the progression of the chemical reaction. The reaction rate is different from one particular point to another point during the progression of a chemical reaction since the reaction rate changes continuously. But the average rate gives the average rate of all these points, but it does not give any information about the point in between initiation and completion of the reaction.
Average Rate of Change Formula
The average rate of change is given as below.
Average Rate = Δ(concentration of reactant or product) /Δ(time)
The average rate gives only the average rate of the whole reaction, but this average rate is not the actual rate throughout the reaction since the reaction rate decreases with the consumption of reactants.
What is the Difference Between Instantaneous Rate and Average Rate?
Instantaneous Rate vs Average Rate | |
| Instantaneous rate is the rate of a chemical reaction that is measured as the change of the concentration of reactants or products during a known time period. | Average rate is the rate of a chemical reaction that is measured as the change of the concentration of reactants or products during the whole time period of the progression of the chemical reaction. |
| Time | |
| Instantaneous rate is measured for a particular moment or for a very short time period. | Average rate is measured for a longer time period. |
| Formula for Calculation | |
| Instantaneous Rate of Change = Lim (t→0) [Δ(concentration of reactant or product) /Δ(time)] | Average Rate of Change = Δ(concentration of reactant or product) /Δ(time) |
Summary – Instantaneous Rate vs Average Rate
Reaction rate of a chemical reaction is the rate of change of concentration of either reactants or products. Reaction rate can be determined in two forms as instantaneous rate and average rate. The main difference between instantaneous rate and average rate is that the instantaneous rate measures the change in concentration of reactants or products during a known time period whereas average rate measures the change in concentration of reactants or products during the whole time take for the completion of the chemical reaction.
Reference:
1. Laidler, Keith J. “Reaction Rate.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 23 Apr. 2014, Available here.
2. “Finding Instantaneous Rate of Change.” Study.com, Available here.
3. Libretexts. “Reaction Rate.” Chemistry LibreTexts, Libretexts, 21 July 2016, Available here.
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau261zayrmqaklrumu9SsZKuZpJp6orrDZq2sZZGrsrOtxp5kq5mkmnw%3D