Difference Between Limiting Reactant and Excess Reactant
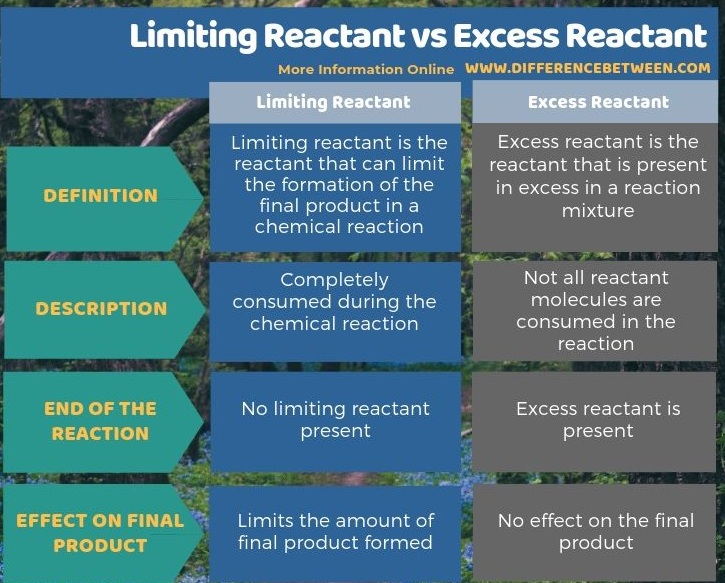
The key difference between limiting reactant and excess reactant is that the limiting reactant can limit the amount of final product produced, whereas excess reactant has no effect on the amount of final product.
A reactant is a compound that is consumed during a chemical reaction. A chemical reaction involves reactants – some reactants in excess and some in limited amounts. The limiting reactant always decides the amount of final product formed after the completion of the reaction. That means, the limiting reactant limits the amount of final product, but there is no such effect by the excess reactant.
CONTENTS
1. Overview and Key Difference
2. What is Limiting Reactant
3. What is Excess Reactant
4. Side by Side Comparison – Limiting Reactant vs Excess Reactant in Tabular Form
5. Summary
What is Limiting Reactant?
Limiting reactant is the reactant of a particular chemical reaction which can limit the formation of the final product. Therefore, it decides how much product we can yield from the completion of the chemical reaction. Moreover, this reactant is completely consumed during the reaction. The reaction stops when all the limiting reactant is consumed. It is because the reaction stops when one reactant is missing.
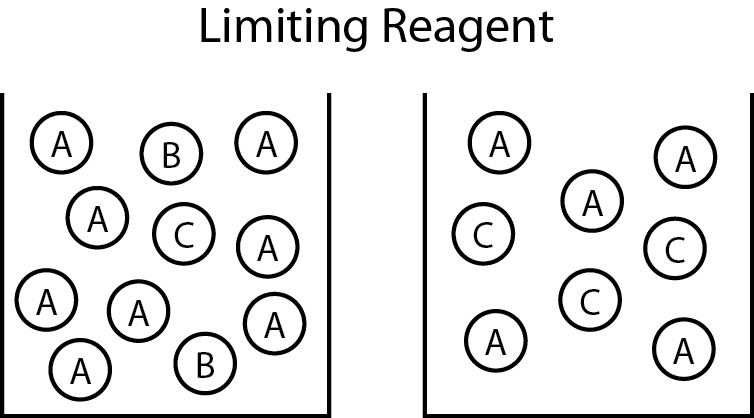
Figure 01: If limiting reactant is B, and the final product is C while excess reactant is A, the final reaction mixture contains A and C.
By looking at the stoichiometric relationship between this reactant and the final product in a chemical equation, we can determine how much product is going to be formed.
What is Excess Reactant?
An excess reactant is the reactant that is present in excess in a reaction mixture. Therefore, after the completion of the reaction, some amount of this reactant still remains since it is in excess. We can observe the presence of excess reactant at the beginning of the reaction, at the progression, and at the end as well. Sometimes the presence of an excess reactant is important in determining an unknown amount of a particular substance that can react with this excess reactant. For example, in titrimetric methods, we use an excess reactant with a known amount and after the completion of the reaction. Here, we can determine the amount of excess reactant still present in the reaction mixture, to determine how much of this reactant reacted with the unknown.
What is the Difference Between Limiting Reactant and Excess Reactant?
The limiting reactant and the excess reactant are important in a chemical reaction. The key difference between limiting reactant and excess reactant is that the limiting reactant can limit the amount of final product produced, whereas excess reactant has no effect on the amount of final product.
Below infographic shows more facts on the difference between limiting reactant and excess reactant.
Summary – Limiting Reactant vs Excess Reactant
The limiting reactant and the excess reactant are important in a chemical reaction. A limiting reactant can limit the amount of final product produced, whereas excess reactant has no effect on the amount of final product. Therefore, this is the key difference between limiting reactant and excess reactant
Reference:
1. “Excess and Limiting Reagents.” Chemistry LibreTexts, Libretexts, 5 June 2019, Available here.
Image Courtesy:
1. “Limiting Reagent” By Brazosport College – Own work (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau264yKagraGenHqzscCcq5qmpGKur7CMnq%2BcnaOoerOxwJyrmqakZA%3D%3D