Difference Between Lindlar and Rosenmund Catalysts
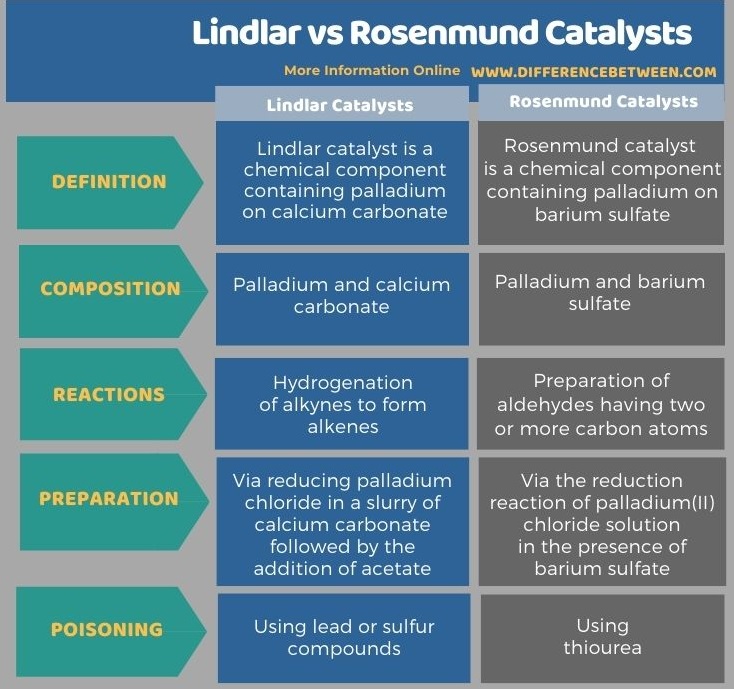
The key difference between Lindlar and Rosenmund catalysts is that Lindlar catalyst contains palladium on calcium carbonate, whereas Rosenmund catalyst contains palladium on barium sulfate.
A catalyst is a chemical compound or a biological component that is useful in reducing the activation energy of a particular chemical reaction in order to increase the rate of reaction. Catalysts are not consumed during the reaction; thus, they are reformed after the completion of the reaction. Lindlar and Rosenmund catalysts are two such chemical compounds.
CONTENTS
1. Overview and Key Difference
2. What are Lindlar Catalysts
3. What are Rosenmund Catalysts
4. Side by Side Comparison –Lindlar vs Rosenmund Catalysts in Tabular Form
5. Summary
What are Lindlar Catalysts?
Lindlar catalyst is a chemical component containing palladium on calcium carbonate. We can categorize it as a heterogeneous catalyst because it contains two components acting as a single unit (palladium + calcium carbonate). This catalyst is produced via depositing palladium on calcium carbonate, which is then poisoned using various forms of lead or sulfur. Lindlar catalysts are important in chemical reactions such as hydrogenation of alkynes to obtain alkenes. This catalyst compound was named after its founder, Herbert Lindlar.
Lindlar catalyst is available as a commercial product. It can be prepared via reducing palladium chloride in a slurry of calcium carbonate. After this reaction, we need to add lead acetate to this reaction mixture. This is called catalyst poisoning. There are some other methods of catalyst poisoning, such as the use of lead oxide and quinolone. Generally, the content of palladium in Lindlar catalyst is 5%.
When considering the mechanism of action of Lindlar catalyst, it acts by deactivating the palladium sites using lead, which is important in preventing the formation of alkane instead of alkene during the hydrogenation of alkyne to alkene. Therefore, if the reactant compound contains both double bonds and triple bonds, only triple bonds are reduced in the presence of Lindlar catalyst.
What are Rosenmund Catalysts?
Rosenmund catalyst is a chemical component containing palladium on barium sulfate. The chemical reaction we use this catalyst is named as Rosenmund reduction. It is a type of hydrogenation reaction where an acyl chloride selectively undergoes reduction to form an aldehyde. This catalyst was named after its founder, Karl Wilhelm Rosenmund.
In the Rosenmund catalyst, there are two components: palladium and barium sulfate so, we can categorize it as a heterogeneous catalyst. Barium sulfate has a low surface area, which can reduce the activity of palladium. This reduction can prevent the over-reduction process. Usually, this catalyst is added with a poison (catalyst poisoning) in order to reduce the activity of certain acyl chlorides. Originally, thiourea is the poison used for Rosenmund catalyst.
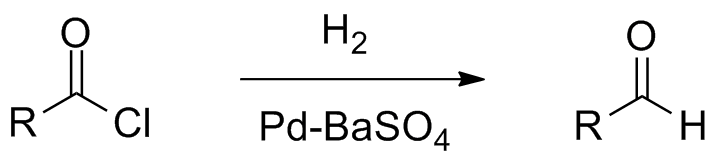
We can prepare a Rosenmund catalyst via the reduction reaction of palladium(II) chloride solution in the presence of barium sulfate. The reducing agent is typically formaldehyde. This catalyst is important in preparing aldehydes, but formaldehyde cannot be prepared because formyl chloride (the acyl chloride) is unstable at room temperature.
What is the Difference Between Lindlar and Rosenmund Catalysts?
Lindlar and Rosenmund catalysts are chemical compounds that are important in enhancing the rate of a chemical reaction by decreasing the activation energy barrier of that reaction. The key difference between Lindlar and Rosenmund catalysts is that Lindlar catalyst contains palladium on calcium carbonate, whereas Rosenmund catalyst contains palladium on barium sulfate.
Below infographic lists more differences between Lindlar and Rosenmund catalysts.
Summary – Lindlar vs Rosenmund Catalysts
Lindlar and Rosenmund catalysts are chemical compounds that are important in enhancing the rate of a chemical reaction by decreasing the activation energy barrier of that reaction. The key difference between Lindlar and Rosenmund catalysts is that Lindlar catalyst contains palladium on calcium carbonate, whereas Rosenmund catalyst contains palladium on barium sulfate.
Reference:
1. “Lindlar Catalyst.” Chemistry LibreTexts, Libretexts, 14 July 2020, Available here.
Image Courtesy:
1. “PhC2HH2” By Smokefoot – Own work (Public Domain) via Commons Wikimedia
2. “Rosenmund Reduction Scheme” By ~K assumed – Own work assumed (based on copyright claims) (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau264yKebpZmiYq6vsIyrpqydnqLCr7CMnJitmZyuwLW%2Fjg%3D%3D