Difference Between Lithium and Other Alkali Metals
The key difference between Lithium and other alkali metals is that the lithium is the only alkali metal that can react with nitrogen whereas the other alkali metals cannot undergo any reaction with nitrogen.
Alkali metals are the group 1 elements of the periodic table of elements. However, it excludes hydrogen because it has nonmetallic properties. Therefore, the chemical elements that we can name as alkali metals include Lithium, Sodium, Potassium, Rubidium, Caesium and Francium. Although lithium is a member of this group, it has some exceptional properties than the other alkali metals such as the ability to react with nitrogen gas.
CONTENTS
1. Overview and Key Difference
2. What is Lithium
3. What are Other Alkali Metals
4. Side by Side Comparison – Lithium vs Other Alkali Metals in Tabular Form
5. Summary
What is Lithium?
Lithium is an alkali metal having the atomic number 3 and chemical symbol Li. According to the big bang theory of the creation of the earth, lithium along with hydrogen and helium are the major chemical elements produced at the earliest stages of the world creation. The atomic weight of this element is 6.941, and the electron configuration is [He] 2s1. Moreover, it belongs to the s block since it is in the group 1 of the periodic table and the melting and boiling points of this element are 180.50 °C and 1330 °C respectively. It appears in silvery-white colour, and if we burn this metal, it gives Crimson coloured flame.

Figure 01: Lithium Metal
This metal is very light and soft. Hence we can cut it simply using a knife. In addition, it can float on water, resulting in an explosive chemical reaction. this metal has some unique properties which other alkali metals do not have. For example, it is the only alkali metal that can react with nitrogen gas and it forms lithium nitride upon this reaction. It is the smallest element among other members of this group. Moreover, it has the least density among solid metals.
Alkali metals are the chemical elements in group 1 of the periodic table of elements except for hydrogen. Thus, the members of this groups that fall into this category are Lithium, Sodium, Potassium, Rubidium, Caesium and Francium. The reason why we name them as alkali metals is that they form alkali compounds.
Figure 02: Alkali Metals in Red Colour
When considering the electron configuration of them, they have their outermost electron in an s orbital; hence they are in the s block of the periodic table. The most stable charged species that they form is the monovalent cation.
Lithium is an alkali metal having the atomic number 3 and chemical symbol Li whereas alkali metals are the chemical elements in group 1 of the periodic table of elements except for hydrogen. The key difference between Lithium and other alkali metals is that the lithium is the only alkali metal that can react with nitrogen whereas the other alkali metals cannot undergo any reaction with nitrogen. Moreover, lithium cannot form an anion while other alkali metals can form anions.
The below infographic tabulates the difference between Lithium and other alkali metals as a side by side comparison.
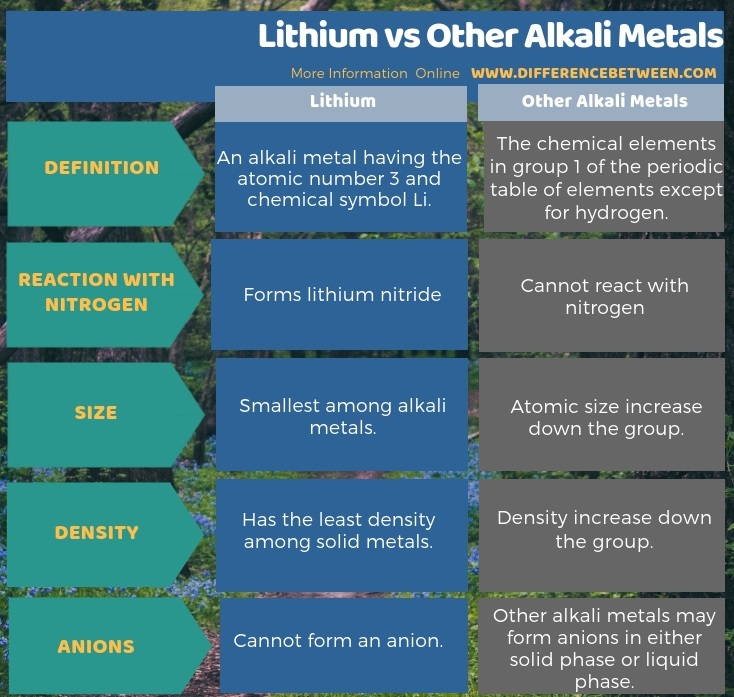
Summary – Lithium vs Other Alkali Metals
Lithium is a member of the group of alkali metals. The key difference between Lithium and other alkali metals is that the lithium is the only alkali metal that can react with nitrogen whereas the other alkali metals cannot undergo any reaction with nitrogen.
Reference:
1. “Lithium.” Wikipedia, Wikimedia Foundation, 30 Sept. 2018. Available here
2. “Alkali Metal.” Wikipedia, Wikimedia Foundation, 11 Sept. 2018. Available here
Image Courtesy:
1.”Lithium paraffin”By Tomihahndorf (Public Domain) via Commons Wikimedia
2.”Periodic table of elements”By 2012rc – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau264yK2foq2dYq6vsIyoq6GdomKurbfApaBmpZWprq2%2Fjg%3D%3D