Difference Between Magnesium Glycinate and Citrate
Key Difference – Magnesium Glycinate vs Citrate
Magnesium glycinate and Magnesium citrate are mainly used as dietary supplements of magnesium. Magnesium glycinate is a magnesium salt of glycine. Magnesium citrate is a magnesium salt of citric acid. These compounds have similarities as well as differences. The key difference between Magnesium glycinate and Magnesium citrate is that Magnesium glycinate acts by absorbing to the body in the form of amino acid whereas Magnesium citrate works by attracting water from tissues via osmosis.
CONTENTS
1. Overview and Key Difference
2. What is Magnesium Glycinate
3. What is Magnesium Citrate
4. Similarities Between Magnesium Glycinate and Citrate
5. Side by Side Comparison – Magnesium Glycinate vs Citrate in Tabular Form
6. Summary
What is Magnesium Glycinate?
Magnesium glycinate is magnesium salt of glycine. Glycine is an amino acid. It is categorized as a non-essential amino acid. The chemical formula of this compound is C4H8MgN2O4. The molar mass of Magnesium glycinate is 172.42 g/mol. The Magnesium glycinate molecule contains magnesium cation and glycinate anion in 1:2 ratio. The IUPAC name of this compound is magnesium 2-aminoacetate.
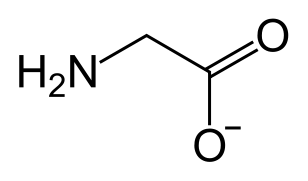
Magnesium glycinate is highly absorbed into our body since it is an amino acid. It can easily be carried to body cells. Hence, this compound is used in magnesium supplements. It is very effective as a supplement because one molecule of Magnesium glycinate contains 14.1% magnesium by weight. Some important benefits of Magnesium glycinate are listed below.
- Using magnesium glycinate can reduce the severe effects of chronic fatigue.
- It also helps in balancing mood swings.
- It acts as a supplement for magnesium deficient patients in helping to decrease high blood pressure (a little).
What is Magnesium Citrate?
Magnesium citrate is magnesium salt of citric acid. The chemical formula of Magnesium citrate is C6H6MgO7. The molar mass of this compound is 214.41 g/mol. The IUPAC name of the compound is magnesium 2-hydroxypropane-1,2,3-tricarboxylate.
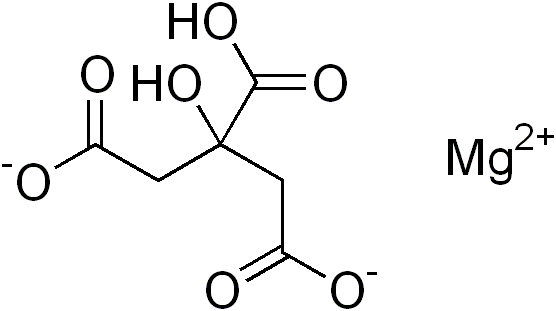
This compound is available as a powder. One molecule of Magnesium citrate contains magnesium cation and citrate anion in the ratio of 1:1. But in some cases, such as trimagnesium citrate compound, it is also known as magnesium citrate. Hence, it is a common term for magnesium salts of citrate. When compared to other forms of magnesium salts citrate, magnesium citrate is more water soluble and is less alkaline.
Most of the times, Magnesium citrate is supplied as a tablet or a liquid for oral use. The commercially available forms of Magnesium citrate are known as Citrate of Magnesia, Citroma, etc. Magnesium citrate is used as a supplement of magnesium. Apart from using it as a dietary supplement, it is used as a food additive as well.
Magnesium citrate works by attracting water from tissues via osmosis. When it is in the intestine, it can attract enough water that is helpful in avoiding constipation, bowel irregularity or bowel evacuation. However, there are some side effects reported. Ex: diarrhoea, abdominal cramps, electrolytic imbalance inside the body, vomiting, etc.
What are the Similarities Between Magnesium Glycinate and Citrate?
- Both Magnesium Glycinate and Citrate are used as medication.
- Both Magnesium Glycinate and Citrate are used as dietary supplements.
- Both Magnesium Glycinate and Citrate are used to treat magnesium deficiency.
What is the Difference Between Magnesium Glycinate and Citrate?
Magnesium Glycinate vs Citrate | |
| Magnesium glycinate is magnesium salt of glycine. | Magnesium citrate is magnesium salt of citric acid. |
| Parent Compound | |
| Magnesium glycinate is a derivative of an amino acid; glycine. | Magnesium citrate is a derivative of an acid; citric acid. |
| IUPAC Name | |
| The IUPAC name of Magnesium glycinate is magnesium 2-aminoacetate. | The IUPAC name of Magnesium citrate is magnesium 2-hydroxypropane-1,2,3-tricarboxylate. |
| Chemical Formula | |
| The chemical formula of Magnesium glycinate is C4H8MgN2O4. | The chemical formula of Magnesium citrate is C6H6MgO7. |
| Molar Mass | |
| The molar mass of Magnesium glycinate is 172.42 g/mol. | The molar mass of Magnesium citrate is 214.41 g/mol. |
| Cation to Anion Ratio | |
| The cation to anion ratio in Magnesium glycinate is 1:2. | The cation to anion ratio in Magnesium citrate is 1:1. |
Summary – Magnesium Glycinate vs Citrate
Magnesium glycinate and Magnesium citrate are compounds derived from different parent compounds by forming magnesium salt of those compounds. Both these compounds are very important as magnesium supplements and are used as medication as well. The difference between Magnesium glycinate and Magnesium citrate is that Magnesium glycinate acts by absorbing into the body in the form of amino acid whereas Magnesium citrate works by attracting water from tissues via osmosis.
Reference:
1.“Magnesium glycinate.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine. Available here
2.“Magnesium citrate.” Wikipedia, Wikimedia Foundation, 17 Feb. 2018. Available here
3.Kent, Linda Tarr. “What Are the Benefits of Magnesium Glycinate?” COM, Leaf Group, 3 Oct. 2017. Available here
Image Courtesy:
1.’Glycinate’By Fvasconcellos (Public Domain) via Commons Wikimedia
2.’Magnesium citrate’By Edgar181 – Own work, (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265wKClnquZqrpus8uymqKmkamybq3NnWSvq12YtrW%2BwK2caA%3D%3D