Difference Between Maillard Reaction and Caramelization
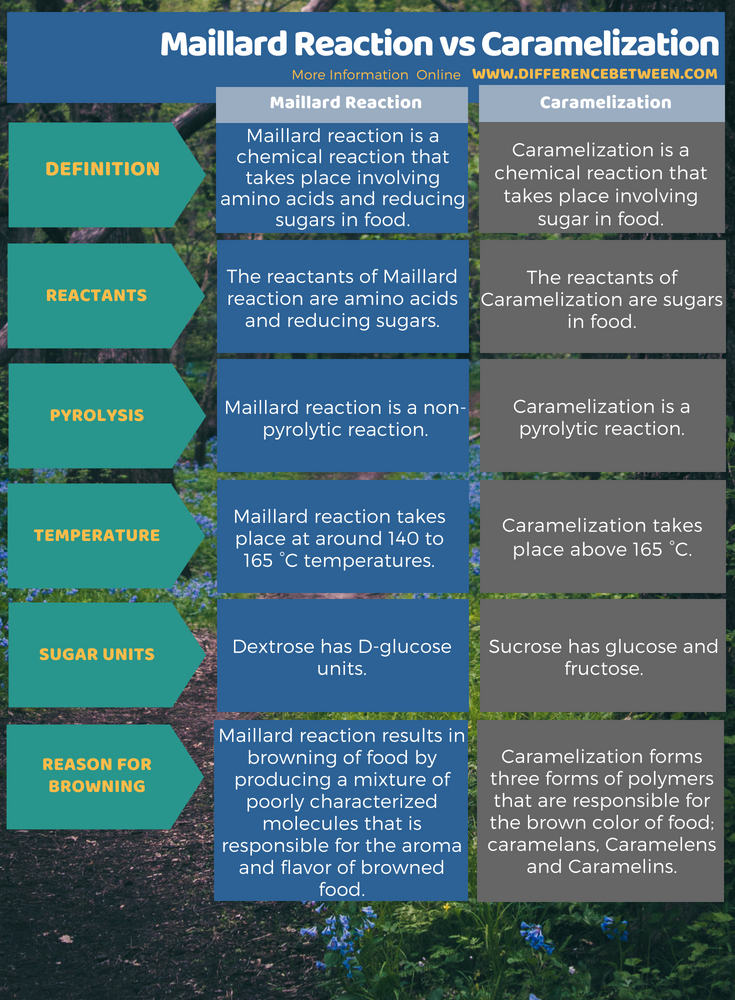
The key difference between Maillard reaction and caramelization is that the Maillard reaction is non-pyrolytic whereas the caramelization is pyrolytic.
The Maillard reaction and caramelization are two different non-enzymatic browning processes of food. These processes, however, differ from each other according to the method of processing. In both cases, the food that undergoes these processes get a brown color at the end of the process.
CONTENTS
1. Overview and Key Difference
2. What is Maillard Reaction
3. What is Caramelization
4. Side by Side Comparison – Maillard Reaction vs Caramelization in Tabular Form
5. Summary
What is Maillard Reaction?
Maillard reaction is a chemical reaction that takes place involving amino acids and reducing sugars in food. This process results in a browned food having a distinctive flavor. It is not an enzyme-catalyzed reaction. Typically, this process occurs at around 140 to 165 °C temperatures. Most of the times, we tend to go for even higher temperatures in order to make sure this reaction has taken place. However, very high temperatures will result in caramelization rather than this reaction.
In Maillard reaction, the carbonyl group of the sugar reacts with the amino group of the amino acid. It results in a mixture of poorly characterized molecules. This mixture of molecules is responsible for the aroma and flavor of browned food.

Figure 01: Browning of Meat
The reaction rate speeds up if we do this in an alkaline environment. This is because there the amino groups tend to deprotonate. This deprotonation increases the nucleophilicity of food. The type of amino acid determines the final flavor.
Examples where we use the Maillard reaction:
- Coffee roasting
- Chocolate production
- Browning of various meats like steak
- The dark crust of baked food
- Production of malted barley
What is Caramelization?
Caramelization is a chemical reaction that takes place involving sugar in food. Therefore we can define it as browning of sugar. This process gives the food its sweet, nutty flavor and brown color while cooking. There are three polymer groups which are responsible for the brown color of the food. they are;
During the progression of this process, some components of the food releases which are highly volatile. For example, it releases diacetyl components of food. this produces the characteristic caramel flavor of food. Moreover, this process is pyrolytic. This means the process involves the thermal decomposition of materials in food.

Figure 02: Caramelization of Carrots
There are many types of chemical reactions that take place during this process. Some of them are as follows:
- Condensation reactions
- Intramolecular bonding
- Unsaturated polymer formations
- Dehydration reactions
- Inversion of sucrose into fructose and glucose
Some examples where we use Caramelization:
- Production of caramel candies
- Making caramelized onions, potatoes, pear, etc.
- Producing caramel sauce, cola products, caramelized sweetened milk, etc.
What is the Difference Between Maillard Reaction and Caramelization?
Maillard reaction is a chemical reaction that takes place involving amino acids and reducing sugars in food. Therefore the reactants of this reaction are amino acids and reducing sugars. Moreover, it is a non-pyrolytic reaction. Here, the browning occurs via producing a mixture of poorly characterized molecules that is responsible for the aroma and flavor of browned food. Caramelization is a chemical reaction that takes place involving sugar in food. hence the reactants of Caramelization are sugars in food. It is a pyrolytic reaction. In addition to that, it forms three forms of polymers that are responsible for the brown color of food; caramelans, Caramelens and Caramelins. The below infographic presents more details on the difference between Maillard reaction and caramelization in tabular form.
Summary – Maillard Reaction vs Caramelization
The difference between Maillard reaction and caramelization is that the Maillard reaction is non-pyrolytic whereas the caramelization is pyrolytic. This means, caramelization involves the thermal decomposition of materials in food (sugar), while Maillard reaction does not involve any thermal decomposition; it occurs via a chemical reaction between amino acids and reducing sugars in food.
Reference:
1. “Maillard Reaction.” Wikipedia, Wikimedia Foundation, 3 Aug. 2018. Available here
2. “Caramelization.” Wikipedia, Wikimedia Foundation, 27 July 2018. Available here
Image Courtesy:
1.”617430″ by 738020 (CC0) via pixabay
2.”Caramelisation of carrots” (CC BY-SA 2.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265wKKjpZmimXqzscCcq6KnnmKur7CMnJirmZ2auarGwK2gqKZf