Difference Between Metal and Alloy
The key difference between metal and alloy is that the metal is a pure substance whereas the alloy is a mixture of two or more components.
We can divide all elements into metals and nonmetals on the basis of certain characteristics. Metal has a lustre, and they are good conductors of heat and electricity. When we polish metals, they are also good reflectors of light. However, it is not just metal but also their solid mixtures, namely alloys, which are very useful for mankind. To a common man, there is no difference between metal and alloy, but there are many differences between these two, and we will highlight them in this article.
CONTENTS
1. Overview and Key Difference
2. What is Metal
3. What is Alloy
4. Side by Side Comparison – Metal vs Alloy in Tabular Form
5. Summary
Metal is a material that has metallic properties. That means, metals have a lustre and are good conductors of heat and electricity. Furthermore, if we polish the surface, they are also good reflectors of light. Also, most of the metals are ductile and malleable. Moreover, they are denser than the non-metals. Most of the times, these materials have higher densities and melting and boiling points. The malleability and ductility of metals enable them to deform under stress without cleaving.

Figure 01: Iron is an Important Metal
Besides, there are three major crystal structures that metal can have;
Above all these, the metals tend to form cations. They form cations via losing electrons from their outermost atomic orbitals. Hence, most of the metals can form oxides with the reaction with oxygen in normal air. However, there are some metals that do not react with air at all due to their high stability.
What is Alloy?
Alloy is a substance that has two or more components mixed with metal. Therefore, it also has metallic properties. Also, an alloy can have either a fixed composition or variable compositions. Looking at the purpose, we make alloys in order to enhance the existing properties of a metal or to provide the metal with new properties. Mostly, the aim of producing alloys is to make them less brittle, harder, resistant to corrosion, or have more desirable colour and lustre. Moreover, it is possible to change the properties of an alloy to suit one’s requirements by varying the additives or alloying materials.
The word alloying has come to refer to the process that leads to the formation of alloys. For centuries, people used iron, thinking it to be very strong. But, it was the formation of steel; its alloy, that gave the world one of the strongest structural materials. Also, there are two major types of alloy as substitutional alloys and interstitial alloys.
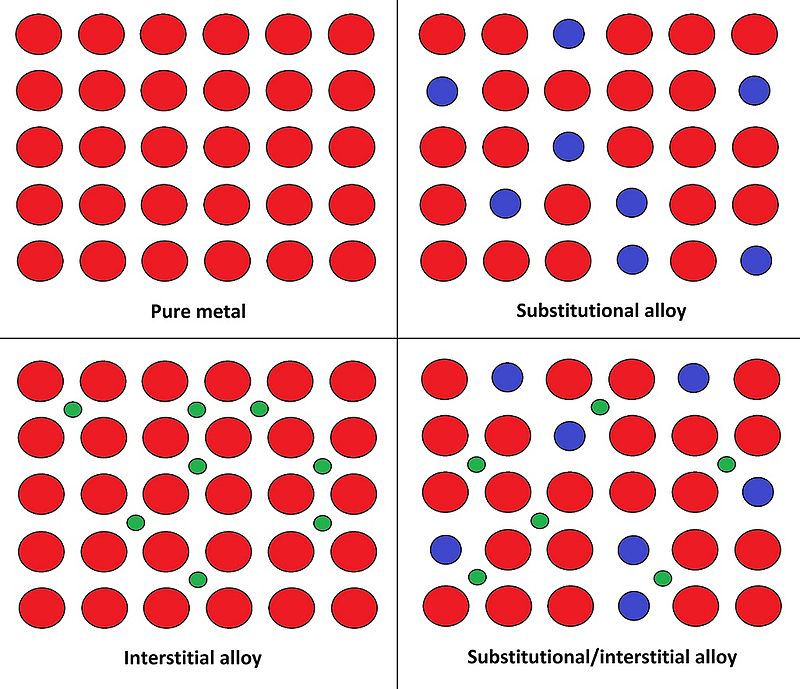
Figure 02: Different types of Alloys
Looking at the example of steel; it is an alloy that consists of mostly iron and a little bit of carbon, whose percentage varies from 0.2% to 2% depending upon the grade of alloy. We are aware of the strength and durability of steel, which is much more than iron which is softer than steel. Therefore, it is clear that by alloying, we can get better materials, and importantly, with properties other than those of the ingredients of the alloy. Furthermore, iron is one metal that makes many alloys other than steel with substances such as manganese, chromium, vanadium, and tungsten etc.
Metal is a material that has metallic properties whereas alloy is a substance which has two or more components mixed with metal. Hence, this is the basic difference between metal and alloy. Moreover, metals are pure substances unless they do not react with air and water but alloy is always a mixture of two or more components. Therefore, metal is a natural substance while alloy is a man-made substance. Another difference between metal and alloy is that, unlike pure metals, alloy does not easily undergo chemical reactions with air and water, which is why we tend to use alloys in car wheels rather than pure metal.
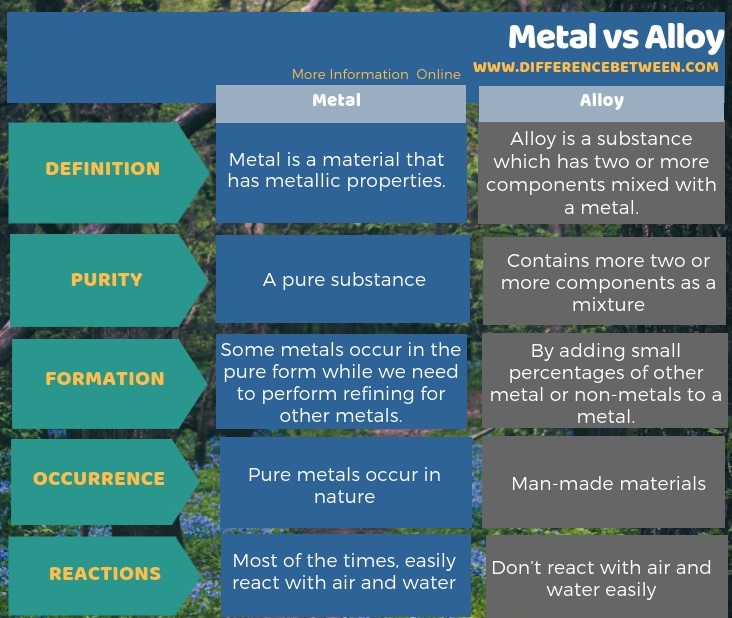
Summary – Metal vs Alloy
Metals are very important substances that we use in our day to day life. Alloy is a subcategory of metal. The key difference between metal and alloy is that the metal is a pure substance whereas the alloy is a mixture of two or more components.
Reference:
1. “Metal.” Wikipedia, Wikimedia Foundation, 4 Oct. 2018. Available here
Image Courtesy:
1.”Iron electrolytic and 1cm3 cube”By Alchemist-hp – Own work, (FAL) via Commons Wikimedia
2.”Alloy atomic arrangements showing the different types”By Zaereth – Own work, (CC0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265xK2YpWWRo7FuwtJmmKWkn658