Difference Between Metal Carbonate and Metal Hydrogen Carbonate
The key difference between metal carbonate and metal hydrogen carbonate is that metal carbonates contain a metal cation and a carbonate anion while metal hydrogen carbonates contain a metal cation and a bicarbonate anion.
Metal carbonate and metal hydrogen carbonate are inorganic compounds. Metal carbonates contain metal cations because a carbonate ion has -2 electrical charge. Metal hydrogen carbonates or metal bicarbonates contain metal cations because the bicarbonate anion has -1 electrical charge.
CONTENTS
1. Overview and Key Difference
2. What is Metal Carbonate
3. What is Metal Hydrogen Carbonate
4. Side by Side Comparison – Metal Carbonate vs Metal Hydrogen Carbonate in Tabular Form
5. Summary
Metal carbonates are inorganic compounds containing a metal cation and a carbonate anion. Carbonate, in chemistry, is a salt of carbonic acid. Carbonate ion is a polyatomic ion having the chemical formula CO32-. The compound that forms from the association of carbonate anion and metal cation is named as a carbonate salt.
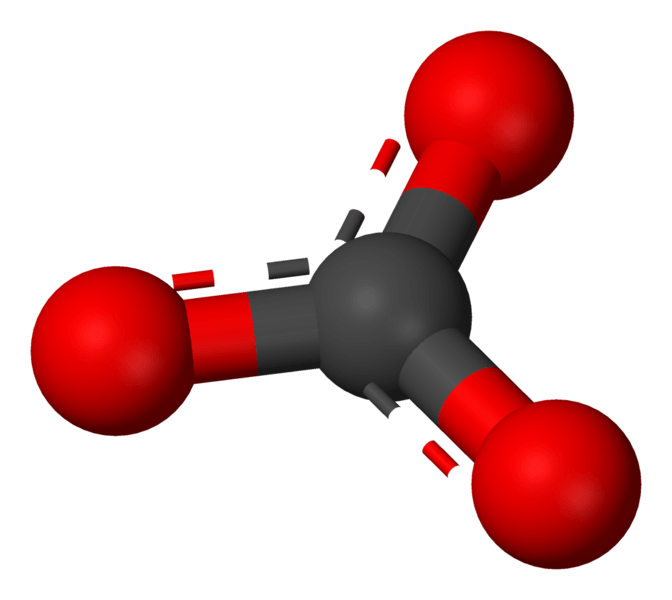
Figure 01: Structure of Carbonate Anion
Generally, metal carbonate compounds undergo decomposition on heat treatment. The metal carbonate tends to liberate carbon dioxide, leaving behind an oxide compound of the metal. We can call this process calcination; this name is derived from the Latin word “calx” used for quicklime or calcium oxide, CaO, which we can obtain from the roasting process of limestone in a lime kiln.
Metal carbonates form when positively charge metal ions interact with carbonate anions. The metal ions or positively charged metal ions can be in the form of M+, M2+, and M3+. These metal ions have monovalent, divalent and trivalent metal ions, respectively. They can associate with negatively charged oxygen atoms in the carbonate anion via the electrostatic attraction forces between the metal ion and carbonate anion. This interaction forms the metal carbonate ionic compound.
Generally, metal carbonates are insoluble in water at standard temperature and pressure conditions. However, there are some exceptions, such as lithium, sodium, and potassium carbonates. Although most metal carbonates are insoluble in water, most bicarbonates are water-soluble compounds.
Metal hydrogen carbonates or metal bicarbonates are inorganic compounds containing a metal cation and a bicarbonate anion. The chemical formula of bicarbonate anion is HCO3–. Bicarbonate is the common name for these compounds while hydrogen carbonates are the IUPAC nomenclature recommended name. According to this nomenclature method, the prefix “bi-“ refers to the presence of a single hydrogen ion. A similar example is bisulfite, where the anion is HSO3-.
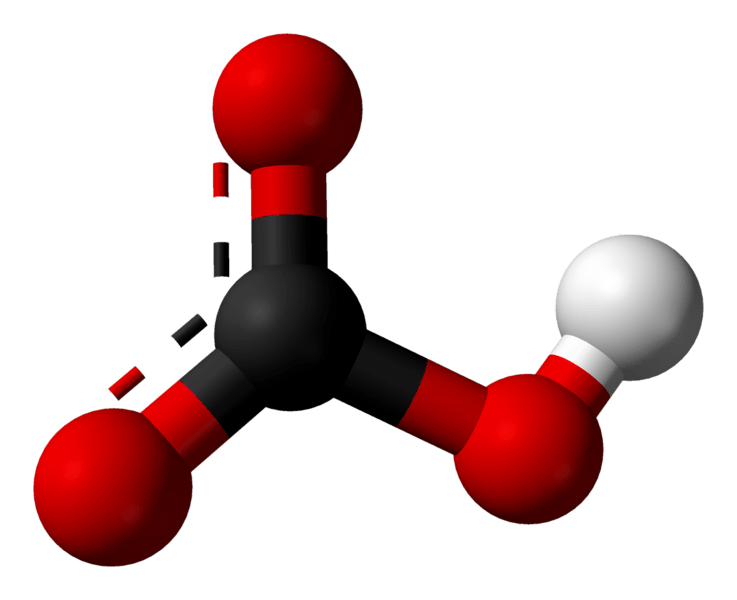
Figure 02: Structure of Bicarbonate Anion
The metal hydrogen carbonate compounds form when positively charged metal cations associate with negatively charged bicarbonate anion. This is an electrostatic attraction where an ionic compound forms. Many bicarbonate compounds are water-soluble at standard temperature and pressure conditions.
Metal carbonate and metal hydrogen carbonate are inorganic compounds. The key difference between metal carbonate and metal hydrogen carbonate is that metal carbonates contain carbonate anions whereas metal hydrogen carbonate compounds contain bicarbonate anions. Most metal carbonates are insoluble in water while most metal hydrogen carbonates are water-soluble compounds. Furthermore, metal carbonate acts as a buffer in blood while metal hydrogen carbonate acts as a component in a pH buffering system in the human body.
Below infographic summarizes the differences between metal carbonate and metal hydrogen carbonate in tabular form.
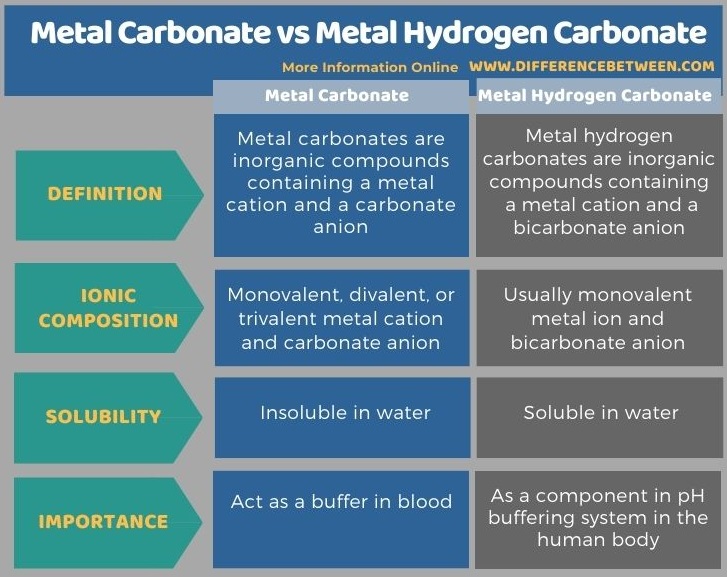
Summary – Metal Carbonate vs Metal Hydrogen Carbonate
Metal carbonate and metal hydrogen carbonate are inorganic compounds. The key difference between metal carbonate and metal hydrogen carbonate is that metal carbonates contain carbonate anions whereas metal hydrogen carbonate compounds contain bicarbonate anions.
Reference:
1. “Bicarbonate.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., Available here.
2. “Carbonate.” Wikipedia, Wikimedia Foundation, 11 Oct. 2020, Available here.
Image Courtesy:
1. “Carbonate-3D-balls” By Benjah-bmm27 – Own work (Public Domain) via Commons Wikimedia
2. “Bicarbonate-ion-3D-balls” By Ben Mills and Jynto – Recolour of File:Nitric-acid-3D-balls-B.png (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau265xK2YpWWTlr%2Bju82aq55lkaOxbrnErZilZZiusbO7xp6lZpuRp6%2BwusCtnGg%3D