Difference Between Multiplicity and Bond Order
The key difference between multiplicity and bond order is that multiplicity refers to the number of possible orientations of the spin of energy level, whereas bond order refers to a measurement of the number of electrons in chemical bonds.
Multiplicity and bond order are properties of chemical compounds. The concept of multiplicity is important in quantum chemistry, while the concept of bond order is important in molecular dynamics.
CONTENTS
1. Overview and Key Difference
2. What is Multiplicity
3. What is Bond Order
4. Side by Side Comparison – Multiplicity vs Bond Order in Tabular Form
5. Summary
What is Multiplicity?
Multiplicity refers to the number of possible orientations of the spin of energy level. This concept is useful in spectroscopy and quantum mechanics. The equation for the measurement of the multiplicity is 2S+1 where “S” refers to the total spin angular momentum. The values we can obtain for the multiplicity includes 1, 2, 3, 4… we can name these as singlets, doublets, triplets, quartets, etc.
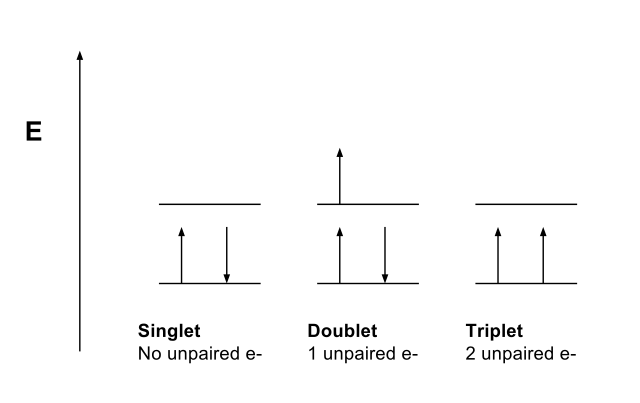
Multiplicity is measured relative to orbital angular momentum. That means; it is measured relative to the number of nearly degenerate energy levels, which are different from each other according to the spin-orbit interaction energy. For example, stable organic compounds have complete electron shells which have no unpaired electrons. Therefore, these molecules have singlet, ground state.
What is Bond Order?
Bond order refers to a measurement of the number of electrons in chemical bonds. The concept of bond order was developed by Linus Pauling. It is useful as an indicator of the stability of a chemical bond. Higher the value of bond order, stronger the chemical bond. If there are no antibonding orbitals, the bond order equals the number of bonds between two atoms of a molecule. This is because the bond order is then equal to the number of bonding electrons divided by two (chemical bonds have two electrons per bond). The equation for the calculation of bond order in a particular molecule is as follows:
Bond order = (number of bonding electrons – number of antibonding electrons)/2
According to the above equation, if the bond order is zero, the two atoms are not bonded with each other. For example, the bond order for dinitrogen molecule is 3. Moreover, the isoelectronic species usually have the same bond order. Apart from that, the concept of bond order is useful in molecular dynamics and bond order potentials.
What is the Difference Between Multiplicity and Bond Order?
The concept of multiplicity is important in quantum chemistry, while the concept of bond order is important in molecular dynamics. The key difference between multiplicity and bond order is that multiplicity refers to the number of possible orientations of the spin of energy level, whereas bond order refers to a measurement of the number of electrons in chemical bonds.
The equation for the determination of multiplicity is 2S+1 where S is the total spin angular momentum. The equation for the determination of bond order is (bonding electrons + antibonding electrons)/2. Moreover, the multiplicity is measured as a relative value (which is relative to the orbital angular momentum). But, the bond order is a particular value for a particular chemical bond. Usually, if the bond order is zero, it means there is no chemical bond.
Below infographic summarizes the difference between multiplicity and bond order.
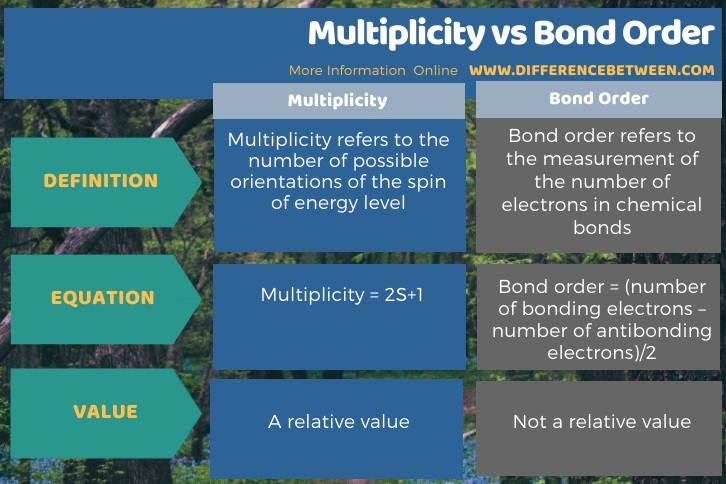
Summary – Multiplicity vs Bond Order
The concept of multiplicity is important in quantum chemistry, while the concept of bond order is important in molecular dynamics. The key difference between multiplicity and bond order is that multiplicity refers to the number of possible orientations of the spin of energy level whereas bond order refers to a measurement of the number of electrons in chemical bonds.
Reference:
1. Helmenstine, Anne Marie. “Bond Order Definition and Examples.” ThoughtCo, Nov. 5, 2019, Available here.
Image Courtesy:
1. “Spin multiplicity diagram” By Llightex – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2651KWroqicnrCqwNhmmKecXZe8r7CMqKmdnaJk