Difference Between Organic and Inorganic Sulfur
The key difference between organic and inorganic sulfur is that organic sulfur refers to the sulfur present in organic compounds, which is highly immobile in the soil, whereas inorganic sulfur refers to the sulfur present in inorganic compounds, which is highly mobile in the soil.
Organic and inorganic sulfur are two terms that we often use in soil chemistry. Sulfur can occur in soil in two forms as organic and inorganic sulfur, depending on the type of compound to which the sulfur atoms are attached. These sulfur-containing compounds circulate through soil systems via different methods such as mobilization, immobilization, mineralization, oxidation and reduction.
CONTENTS
1. Overview and Key Difference
2. What is Organic Sulfur
3. What is Inorganic Sulfur
4. Side by Side Comparison – Organic vs Inorganic Sulfur in Tabular Form
5. Summary
What is Organic Sulfur?
The term organic sulfur refers to the sulfur atoms present in organic compounds. These are sulfur-containing compounds we can observe in soil. These organic sulfur compounds are mostly immobile. There are two major forms of organic sulfur in the soil; they are ester sulfates and carbon-bonded sulfur. Ester sulfates have characteristic linkages which have the general chemical formula C-O-SO3. In directly carbon-bonded organic sulfur compounds, we can observe the chemical bond –C-S. however, there are few other organic sulfur forms as well, but they are not analyzed in detail because they are not that much important in soil chemistry.
There are different types of ester sulfates, such as choline sulfate, phenolic sulfate, sulfated polysaccharides, etc. Examples for carbon-bonded sulfur compounds include amino acids and sulpholipids.
Generally, ester sulfates form from microbial biomass materials and other materials formed via microbial action. These ester sulfates are stored as readily available sulfur. When microbes or plants require sulfur, it is released as quickly as possible. Plant roots and microbes then hydrolyze these organic sulfur compounds to obtain sulfur atoms required.
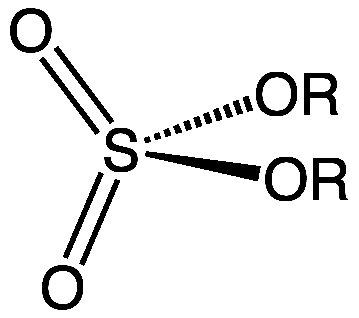
Figure 01: General Structure for Ester Sulfate
When considering the directly carbon-bonded sulfur compounds, they form from litter and dead root parts. Some of these compounds are present in microbial biomass as well. The breakdown of these compounds is difficult compared to ester sulfates. Therefore, they are less available for plants and microbial nutrition.
What is Inorganic Sulfur?
Inorganic sulfur refers to the sulfur atoms present in inorganic compounds. These compounds are mobile in soil systems. Inorganic sulfur mainly occurs in the atmosphere, in different gaseous forms such as hydrogen sulfide, sulfur dioxide, etc.
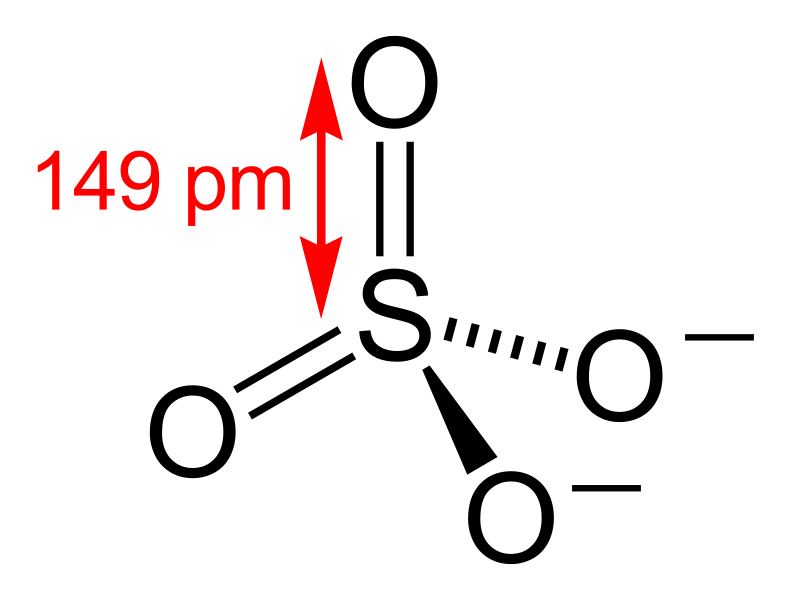
Figure 02: Sulfate Anion
In soil systems, these compounds are mainly salts containing sulfate anion. Sulfate anion is the most mobile form in the soil. Moreover, elemental sulfur and sulfides are uncommon in soil systems.
What is the Difference Between Organic and Inorganic Sulfur?
Organic and inorganic sulfur-containing compounds can be observed in soil. The term organic sulfur refers to the sulfur present in organic compounds, whereas the term inorganic sulfur refers to the sulfur present in inorganic compounds. Moreover, organic sulfur is highly immobile in the soil while inorganic sulfur is highly mobile in soil. So, this is the key difference between organic and inorganic sulfur.
Below infographic summarizes the difference between organic and inorganic sulfur.
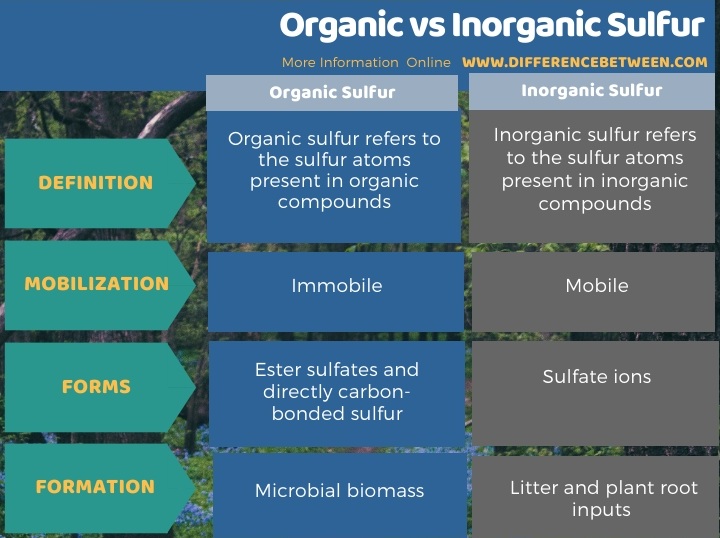
Summary – Organic vs Inorganic Sulfur
Organic and inorganic sulfur-containing compounds can be observed in soil. The key difference between organic and inorganic sulfur is that the term organic sulfur refers to the sulfur present in organic compounds and they are highly immobile in the soil, whereas the term inorganic sulfur refers to the sulfur present in inorganic compounds and they are highly mobile in soil.
Reference:
1. Edwards, Pamela. “Sulfur Cycling, Retention, and Mobility in Soils: A Review.” USDA, Northeastern Research Center, Available here.
Image Courtesy:
1. “SulfateEster” By Smokefoot – Own work (Public Domain) via Commons Wikimedia
2. “Sulfate-ion-2D-dimensions” By YinY. – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2670aCYp6GTYq6vsIyipaiql5a7qq%2BMrKylnqWnfA%3D%3D