Difference Between Oxidation Potential and Reduction Potential
The key difference between oxidation potential and reduction potential is that oxidation potential indicates the tendency of a chemical element to be oxidized. In contrast, reduction potential indicates the tendency of a chemical element to be reduced.
Oxidation potential and reduction potential are two types of electrode potential values for chemical species given in Volts at standard conditions. Therefore, we name them standard oxidation potential and standard reduction potential. The value of these potentials determines the ability of a particular chemical species to undergo oxidation/reduction.
CONTENTS
1. Overview and Key Difference
2. What is Oxidation Potential
3. What is Reduction Potential
4. Side by Side Comparison – Oxidation Potential vs Reduction Potential in Tabular Form
5. Summary
What is Oxidation Potential?
The oxidation potential is a value that indicates the tendency of a chemical species to be oxidized. In other words, it is the ability of an electrode to lose electrons (to get oxidized). Usually, this value is given at standard conditions; hence, we should name it as standard oxidation potential. The denotation for this term is SOP. It is measured in Volts. And, this is very similar to the standard reduction potential, but they are different in the sign of the value, i.e. the value of the standard oxidation potential is the negative value of the standard reduction potential. We can write the oxidation potential as a half-reaction. The general formula for an oxidation reaction and the oxidation potential for copper is given below:

Half reaction of copper oxidation: Cu(s) ⟶ Cu2+ + 2e–
The value for standard oxidation potential for the above reaction (oxidation of copper) is -0.34 V.
What is Reduction Potential
Reduction potential is the tendency of a particular chemical species to undergo reduction. That means; this particular chemical species is willing to accept electrons from outside (to get reduced). It is measured in Volts and usually measured under standard conditions. Therefore, we can name it as standard reduction potential. The denotation for this term is SRP. We can write it in the form of a reduction half-reaction. The general formula and copper as an example are given below:

Half reaction of copper reduction: Cu2+ + 2e– ⟶ Cu(s)
The value for standard reduction potential for the above reaction (reduction of copper) is 0.34 V, which is the exact value, but the opposite sign from that of the oxidation potential of the same chemical species, copper. Therefore, we can develop a relationship between the standard oxidation and reduction potentials as follows:
E00(SRP) = -E00(SOP)
What is the Difference Between Oxidation Potential and Reduction Potential?
Oxidation potential and reduction potential are two types of electrode potential values for chemical species given in Volts at standard conditions. The key difference between oxidation potential and reduction potential is that oxidation potential indicates the tendency of a chemical element to be oxidized, whereas the reduction potential indicates the tendency of a chemical element to be reduced. Since these potential values are measured at standard conditions, we should name them as standard oxidation potential and standard reduction potential.
Moreover, we denote them as SOP and SRP. Furthermore, there is a relationship between these two terms; the standard oxidation potential is the exact same value but with a different sign from that of the standard reduction potential.
Below infographic summarizes the difference between oxidation potential and reduction potential.
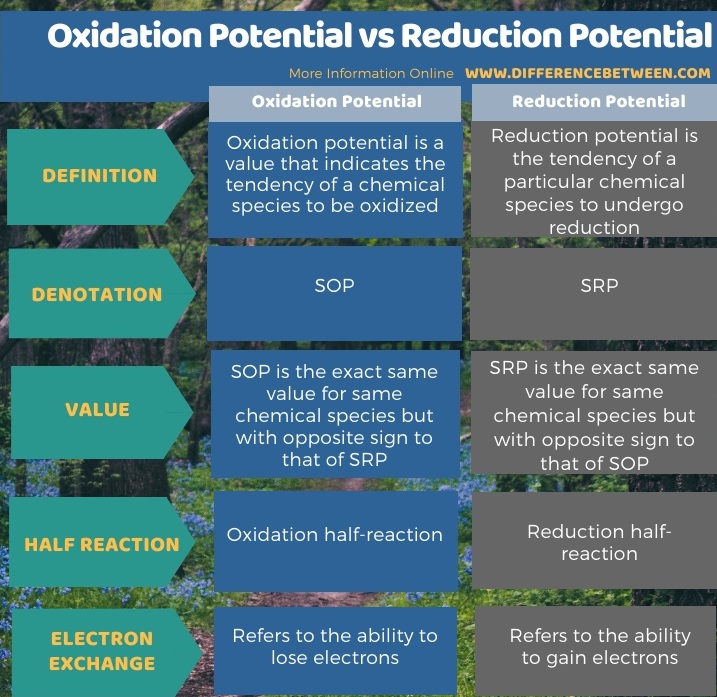
Summary – Oxidation Potential vs Reduction Potential
The oxidation potential and reduction potential are two types of electrode potential values for chemical species given in Volts at standard conditions. The key difference between oxidation potential and reduction potential is that oxidation potential indicates the tendency of a chemical element to be oxidized, whereas the reduction potential indicates the tendency of a chemical element to be reduced.
Reference:
1. “Standard Reduction Potential”. Chemistry Libretexts, 2019, Available here.
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26716KbmqyZpLtuvM6tnKesmZa5bq3NnWSrnZSqsLW1zqdkqaekmru1tcClZg%3D%3D