Difference Between p Alkalinity and m Alkalinity
Key Difference – p Alkalinity vs m Alkalinity
The term alkalinity refers to the amount of an aqueous solution required to neutralize the acidity caused by an acid. Although alkalinity is related to the basicity of an aqueous solution such as water, blood, etc., it measures the resistance of the solution towards the pH changes due to the presence of an acid. The major ions that contribute to the alkalinity of water are Hydroxyl ions (OH–), Carbonate ions (CO32-) and Bicarbonate ions (HCO3-). Alkalinity is categorized into three groups according to the end point given when an aqueous basic solution is titrated with an acid. Caustic Alkalinity, p Alkalinity, and m Alkalinity are these categories. This article focus on the difference between p alkalinity and m alkalinity. The names p Alkalinity and m Alkalinity are given depending on the indicator that is used in the titration process. The key difference between p Alkalinity and m Alkalinity is that p Alkalinity determines alkalinity of all Hydroxyl and half of the Carbonate whereas m Alkalinity determines alkalinity of all Hydroxyl, Carbonate and Bicarbonate. m Alkalinity is considered as the general or total alkalinity because carbonate species play a major role in total alkalinity of water.
CONTENTS
1. Overview and Key Difference
2. What is p Alkalinity
3. What is m Alkalinity
4. Side by Side Comparison – p Alkalinity vs m Alkalinity in Tabular Form
5. Summary
What is p Alkalinity?
The term p Alkalinity stands for “Phenolphthalein – Alkalinity”. It is the measurement of Hydroxide (OH–) and carbonate ion (CO3-2) amount. It is determined by titrating a water sample with an acid of a known concentration in the presence of phenolphthalein as the indicator. To understand what happens in this titration, it is important to know about the dissociation of carbonic acid.
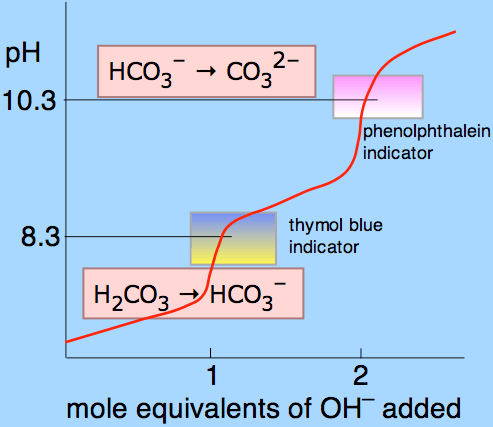
Figure 01: Titration curve for carbonic acid using phenolphthalein and thymol blue as indicators.
The above curve shows what happens during the titration of carbonic acid. It is a diprotic acid and can remove two hydrogen atoms which are called protons. The upper part of the curve indicates that the carbonate and hydroxyl ion amount is given in the pH range of phenolphthalein. Since the pH range where phenolphthalein gives the color change is 8.3 – 10.0, the p Alkalinity is measured in that pH range. Here, the following relationship is used to explain the alkalinity of that particular sample used for titration.
1 mL acid = 1 meq/L alkalinity
What is m Alkalinity?
The total measurement of Hydroxide (OH–), bicarbonate (HCO3–) and carbonate (CO32-) ions amount is given by m Alkalinity. The letter m refers to Methyl orange. It is the indicator that is used to determine the total alkalinity given by the above hydroxide and carbonate species. When methyl orange is added, it gives its color change only in its pH range which is, 3.1 – 4.4. Since only trace concentrations of other acids are dissolved in water except for carbonic acid, m Alkalinity can be considered as the total alkalinity because it gives the total carbonate alkalinity.
What is the difference between p Alkalinity and m Alkalinity?
p Alkalinity vs m Alkalinity | |
| p alkalinity is the measurement of alkalinity given by hydroxide ions and half of the carbonate alkalinity. | m alkalinity is the measurement of alkalinity given by hydroxide ions and total carbonate alkalinity. |
| Indicator | |
| Phenolphthalein indicator is used to determine p alkalinity. | Methyl orange is used to determine m alkalinity. |
| pH Range | |
| p alkalinity is measured at a range of 8.3 – 10.0 pH. | m alkalinity is measured at a pH range of 3.1 – 4.4. |
| Carbonate Species | |
| p alkalinity mainly determines OH– and HCO3– species. | m alkalinity determines OH–, HCO3– and CO32- species. |
Summary – p Alkalinity vs m Alkalinity
By measuring the p alkalinity and m alkalinity, one can calculate the amount of total inorganic carbon that is dissolved in the sample. A number of acids are naturally dissolved in water but in trace concentrations. However, carbonic acid is found in high concentrations because CO2 can dissolve in water. Therefore, the total alkalinity of water is often equal to carbonate alkalinity. The main difference between p Alkalinity and m Alkalinity is that p Alkalinity is the measurement of alkalinity given by hydroxide ions and half of the carbonate alkalinity whereas m alkalinity is the measurement of alkalinity given by hydroxide ions and total carbonate alkalinity.
Download PDF Version of p Alkalinity vs m Alkalinity
You can download PDF version of this article and use it for offline purposes as per citation notes. Please download PDF version here Difference Between p Alkalinity and m Alkalinity.
Reference:
1.”Titration of a Weak Polyprotic Acid.” Libre Text Chemistry. N.p., 6 Jan. 2016. Web. Available here. 5 June 2017.
Image Courtesy:
1. “Titcurve H2CO3” By Slower – Own work (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau268jJqjpJmcnruqwNhmmKecXavAbrmMmqOkmZyeu6rA2Gg%3D