Difference Between Paired and Unpaired Electrons
Paired electrons in an atom occur as pairs in an orbital but, unpaired electrons do not occur as electron pairs or couples. The key difference between paired and unpaired electrons is that the paired electrons cause diamagnetism of atoms whereas the unpaired electrons cause paramagnetism or ferromagnetism in atoms.
Electrons are subatomic particles in atoms. Each and every atom contains at least one electron. In the neutral state of an atom, the number of electrons equals the number of protons in the atomic nucleus. But when it has an electrical charge, these numbers are unequal (which cause the electrical charge). We can write the electron configuration for an atom; it gives the arrangement of electrons in different energy levels. This electron configuration reveals about the paired and unpaired electrons in an atom. now let us discuss what these two forms are.
CONTENTS
1. Overview and Key Difference
2. What are Paired Electrons
3. What are Unpaired Electrons
4. Side by Side Comparison – Paired vs Unpaired Electrons in Tabular Form
5. Summary
What are Paired Electrons?
Paired electrons are the electrons in an atom that occur in an orbital as pairs. An orbital is the location of an electron in an atom; rather than a specific location, it gives the region where an electron moves around the atom because electrons are in continuous movement around the atomic nucleus. According to the modern theories, the electrons exist in orbitals. One simplest orbital can hold a maximum of two electrons. When there are two electrons in one orbital, we say there is a pair of electrons. These are the paired electron in an atom. Some chemical elements having all their electrons paired, are very stable. But some are reactive. The stability depends on the electron configuration of the atom.
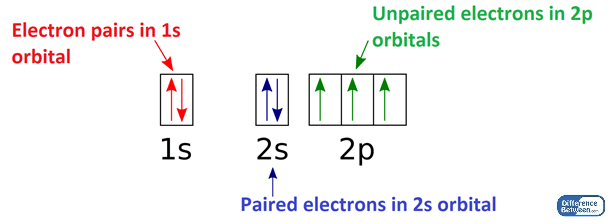
Figure 01: Arrangement of Electrons in Orbitals of Nitrogen Atom
Moreover, if we consider the magnetic properties of a chemical element, there can be three major types of magnetism as diamagnetic, paramagnetic and ferromagnetic elements. This magnetism mainly depends on the number of unpaired electrons. Therefore, the paired electrons have no contribution to the magnetism. Then we can name the chemical elements having all their electrons paired as diamagnetic chemical elements; diamagnetism means it does not attract to a magnetic field.
What are Unpaired Electrons?
Unpaired electrons are the electrons in an atom that occur in an orbital alone. This means these electrons are not paired or occur as electron couples. We can easily determine if there are unpaired electrons in an atom by simply writing its electron configuration. The atoms having these electrons show paramagnetic properties or ferromagnetic properties.
Paramagnetic materials have few unpaired electrons while ferromagnetic materials have more unpaired electrons; thus, ferromagnetic materials attract to a magnetic field at a higher degree than that of a paramagnetic material. When an atom or a molecule has this type of electron, we call it a free radical. The chemical elements having these electrons are highly reactive. This is because they tend to pair all their electrons in order to become stable; having an unpaired electron is unstable.
What is the Difference Between Paired and Unpaired Electrons?
Paired electrons are the electrons in an atom that occur in an orbital as pairs whereas unpaired electrons are the electrons in an atom that occur in an orbital alone. Therefore, paired electrons always occur as a couple of electrons while unpaired electrons occur as single electrons in the orbital. This is the fundamental difference between paired and unpaired electrons. Moreover, the paired electrons cause diamagnetism of atoms whereas the unpaired electrons cause paramagnetism or ferromagnetism in atoms. We can say this as the key difference between paired and unpaired electrons.
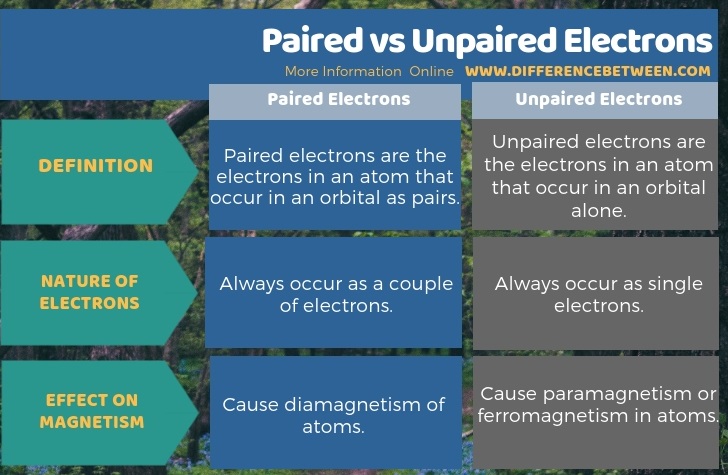
Summary – Paired vs Unpaired Electrons
Electrons occur in atomic orbitals. They are in free movement around the atomic nucleus. These electrons may occur in two types as paired or unpaired electrons. The difference between paired and unpaired electrons is that paired electrons cause the diamagnetism of atoms whereas unpaired electrons cause the paramagnetism or ferromagnetism in atoms.
Reference:
1. “Unpaired Electron.” Wikipedia, Wikimedia Foundation, 3 July 2018. Available here
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau268wKKpnpxdlruledSnp5qhopqxbrHLnpqtqp%2BjwHA%3D