Difference Between Primitive Hexagonal Unit Cell and Hexagonal Closed Packing
The key difference between primitive hexagonal unit cell and hexagonal closed packing is that hexagonal unit cell is the repeating unit of a hexagonal crystal system whereas hexagonal closed packaging is the structure of a crystal lattice having a hexagonal unit cell.
The hexagonal crystal family in crystallography is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). There are 12 point groups in hexagonal crystal family where at least one of their space groups has a hexagonal lattice as the underlying lattice and is the union of the hexagonal crystal system and the trigonal crystal system.
CONTENTS
1. Overview and Key Difference
2. What is Primitive Hexagonal Unit Cell
3. What is Hexagonal Closed Packing
4. Side by Side Comparison – Primitive Hexagonal Unit Cell vs Hexagonal Closed Packing in Tabular Form
5. Summary
What is Primitive Hexagonal Unit Cell?
Primitive hexagonal unit cell is a term that is used to name the repeating unit of a hexagonal crystal lattice. This unit cell is abbreviated as an hcp unit cell. It has a high atomic density because there is a hexagon shape in the cross-section of the crystal lattice.
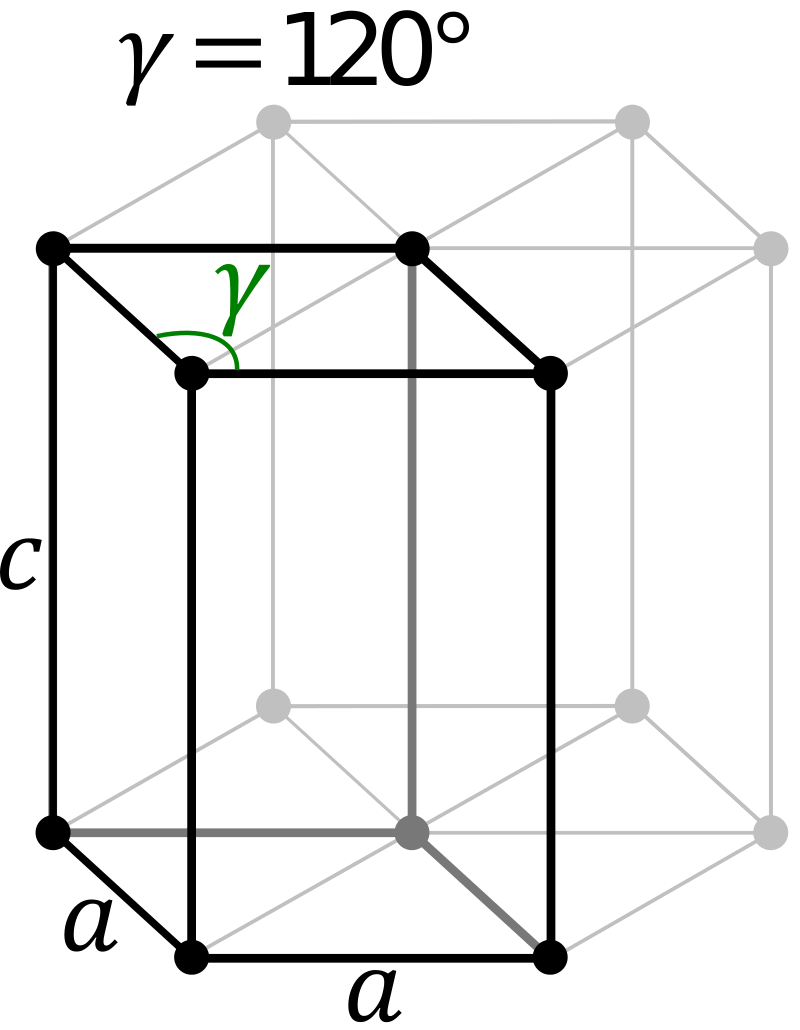
Figure 01: Structure of Primitive Hexagonal Unit Cell
The primitive cell of hexagonal crystal lattice can be described as a right rhombic prism unit cell with two equal axes (named as a and b), an included angle of 120° (named as γ) and a height (named as c, which can be different from a) perpendicular to the two base axes.
What is Hexagonal Close Packing?
Hexagonal close packing is a term that is used to name a crystal lattice with hexagonal unit cells. Hexagonal close packing (HCP) is an arrangement of spheres in a lattice; there are two layers of spheres placed one on the other, forming tetrahedral and octahedral holes. That means; the second layer of spheres are placed in such a way that the trigonal holes of the first layer are covered by the spheres of the second layer. The third layer of spheres resembles the first layer, and the fourth layer resembles the second layer, hence, the structure repeats. Therefore, the repeating unit of a hexagonal close packing arrangement is composed of two layers of spheres.

Figure 02: An Example of a Hexagonal Close Packing Structure is Quartz
Since the same structure repeats after every two layers of spheres, the spheres efficiently fill up 74% of the volume of the lattice. The empty spaces are around 26%. Each sphere in this arrangement is surrounded by 12 neighbouring spheres. When the centres of these 13 spheres (one sphere + 12 neighbouring spheres) are considered, it gives a six-sided pyramid with a hexagonal base. This leads to name this structure as a hexagonal close packing arrangement. The hexagonal close packing arrangement has one large octahedral hole per sphere that is surrounded by six spheres; for each sphere, there are two tetrahedral holes surrounded by four spheres.
What is the Difference Between Primitive Hexagonal Unit Cell and Hexagonal Closed Packing?
The key difference between primitive hexagonal unit cell and hexagonal closed packing is that the term hexagonal unit cell describes the repeating unit of a hexagonal crystal system whereas the term hexagonal closed packaging refers to the structure of the crystal lattice having a hexagonal unit cell.

Summary – Primitive Hexagonal Unit Cell vs Hexagonal Closed Packing
Hexagonal crystal family in crystallography is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). The key difference between primitive hexagonal unit cell and hexagonal closed packing is that the term hexagonal unit cell describes the repeating unit of a hexagonal crystal system whereas the term hexagonal closed packaging refers to the structure of the crystal lattice having a hexagonal unit cell.
Reference:
1. “Hexagonal Crystal Family.” Wikipedia, Wikimedia Foundation, 27 Mar. 2020, Available here.
2. “Closest Packed Structures.” Chemistry LibreTexts, Libretexts, 19 May 2020, Available here.
Image Courtesy:
1. “Hexagonal latticeFRONT” By Bor75 – Own work (CC BY-SA 3.0) via Commons Wikimedia
2. “Quartz crystals on section of limestone core” By Max.kit – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2680aKkoqyZq7JutMSxmKCnnpa5bsHNoqtmm5WhuW6tzZ1koZ2olrSwusClZJykn6iypXnPmpqkoZ6cfA%3D%3D