Difference Between Protein Denaturation and Hydrolysis
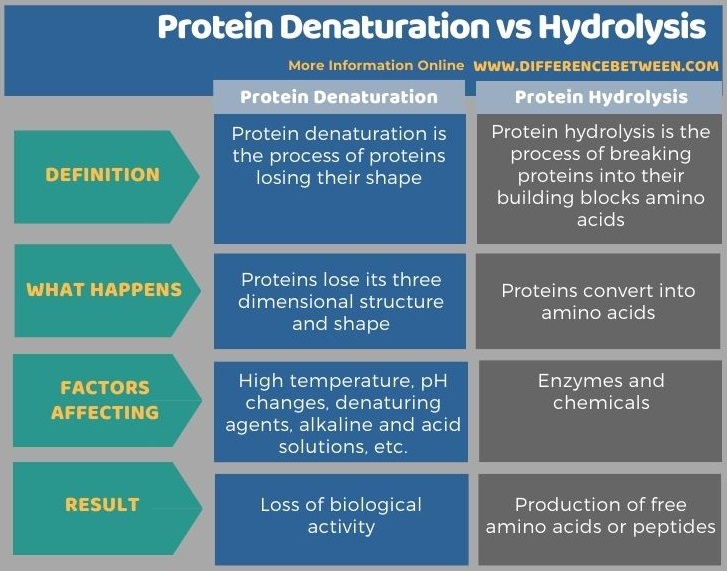
The key difference between protein denaturation and hydrolysis is that in protein denaturation, a protein loses its three-dimensional structure and function while in protein hydrolysis, proteins are mainly converted into their individual amino acids by enzymes.
Proteins are macromolecules composed of amino acids. In a protein, there are hundreds or thousands of amino acids bound to each other. In order to form a functional protein, polypeptide chains interact with each other and form intramolecular bonding to form the protein’s unique three-dimensional structure. The final shape of the protein is most energetically favourable and stable. Moreover, it is biologically active. There are thousands of chemical bonds within the protein 3D structure. Some factors disrupt the three-dimensional structure or the final shape of the protein. Therefore, the protein loses its shape and function. We call it protein denaturation. Protein hydrolysis breaks the protein into its individual amino acids. It is basically done by enzymes.
CONTENTS
1. Overview and Key Difference
2. What is Protein Denaturation
3. What is Protein Hydrolysis
4. Similarities Between Protein Denaturation and Hydrolysis
5. Side by Side Comparison – Protein Denaturation vs Hydrolysis in Tabular Form
6. Summary
What is Protein Denaturation?
Proteins have their unique three-dimensional shapes. Three-dimensional structure is very important for a protein for its specific function. However, due to different causes, protein can lose its shape and function. We call this process protein denaturation. Proteins lose their shape when the bonding and interaction responsible for the secondary and tertiary structures of the protein are disrupted. High temperature, pH changes, certain chemicals and exposure to radiation, etc, can denature proteins. Moreover, proteins can be denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents such as ethanol or acetone. Urea and guanidinium chloride are the two most frequently used denaturing agents.
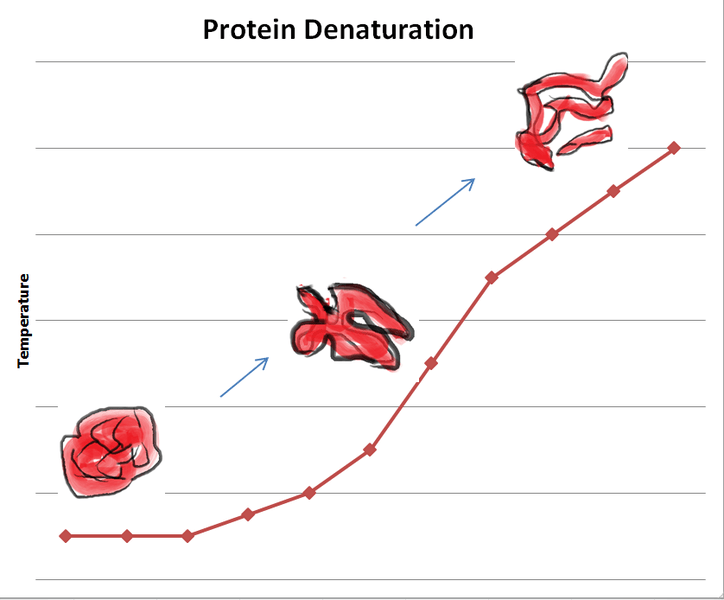
Figure 01: Protein Denaturation
Protein denaturation is irreversible in extreme situations. Rarely, the original structure of the denatured protein can be regenerated. If the primary structure and other factors are intact, there is sometimes a possibility of renaturation (reverse denaturation).
What is Protein Hydrolysis?
Protein hydrolysis is the conversion of proteins into amino acids and peptides. It can be done enzymatically as well as chemically. During hydrolysis, bonds between amino acids are disrupted in order to form free amino acids. Protein hydrolysis takes place naturally within organisms due to enzymes such as pancreatic proteases, etc. When we consume protein-rich food, they are hydrolyzed into small peptides during the digestion process by enzymes.
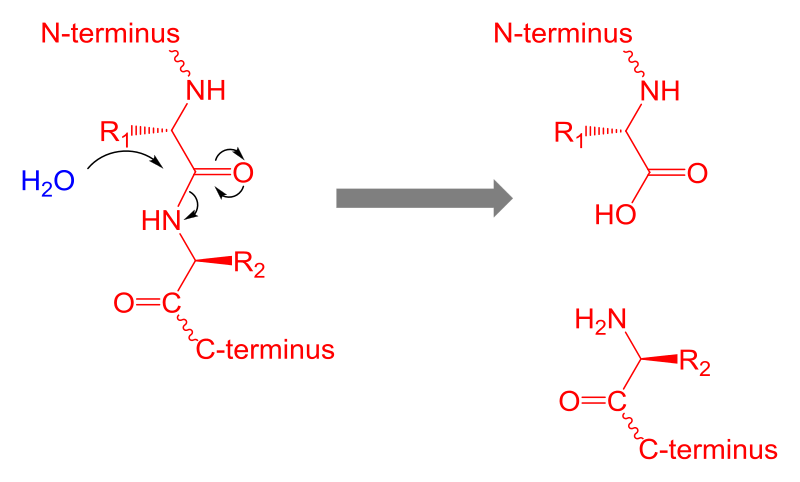
Figure 02: Protein Hydrolysis
Protein hydrolysis is important since it allows isolation of individual amino acids. As an example, histidine can be isolated from red blood cells. Similarly, cystine can be isolated from hydrolysis of hair. In some instances, when it is needed to quantify the total amino acid content in a sample, multiple hydrolysis procedures should be carried out. Acid hydrolysis is the most common technique of protein analysis. Moreover, alkaline hydrolysis can also be employed to measure tryptophan. Therefore, the method of choice for the hydrolysis of proteins depends on their sources.
What are the Similarities Between Protein Denaturation and Hydrolysis?
- Protein denaturation and hydrolysis cause change into protein structure.
- Denaturation often precedes hydrolysis.
- Temperature and pH are two common factors affecting both denaturation and hydrolysis.
What is the Difference Between Protein Denaturation and Hydrolysis?
Protein denaturation makes proteins lose their three-dimensional shape while protein hydrolysis forms free amino acids and peptides. So, this is the key difference between protein denaturation and hydrolysis. Moreover, protein denaturation happens due to high temperature, pH changes, certain chemicals, etc. Protein hydrolysis can be done using enzymes and chemicals.
Below infographic tabulates more differences between protein denaturation and hydrolysis.
Summary – Protein Denaturation vs Hydrolysis
Protein denaturation refers to the disruption of the secondary or tertiary structure of the protein, especially the destruction of alpha-helix and beta sheets. However, the primary structure of the protein remains even after the denaturation. The most common observation when denaturing a protein is the precipitation or coagulation. Protein hydrolysis refers to the conversion of proteins into their amino acids and peptides. It is an important process when isolating individual amino acids. Thus, this summarizes the difference between protein denaturation and hydrolysis.
Reference:
1. “Protein Structure.” Nature News, Nature Publishing Group, Available here.
2. “Protein Denaturation.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., Available here.
Image Courtesy:
1. “Protein Denaturation graph” By Rhannosh – Own work (CC BY-SA 3.0) via Commons Wikimedia
2. “Proteolysis uncat” By Thomas Shafee – Own work (CC BY 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau2680airnqGeYrGmusCtrKuZpJ68r3nAp5tmoKmZv7C42KygrGc%3D