Difference Between Resonance and Mesomeric Effect
The key difference between resonance and mesomeric effect is that the resonance is a result of interaction between lone electron pairs and bond electron pairs whereas the mesomeric effect results due to the presence of substituent groups or functional groups.
The two chemical concepts of resonance and mesomeric effect determine the exact chemical structure of an organic molecule. The resonance arises in molecules having lone electron pairs on any of the atoms in the molecule. The mesomeric effect arises if a molecule has substituents or functional groups. Both these phenomena are common in organic molecules.
CONTENTS
1. Overview and Key Difference
2. What is Resonance
3. What is Mesomeric Effect
4. Side by Side Comparison – Resonance vs Mesomeric Effect in Tabular Form
5. Summary
What is Resonance?
Resonance is a theory in chemistry that describes the interaction between lone electron pairs and bond electron pairs of a molecule. This determines the actual structure of that molecule. We can observe this effect in molecules having lone electron pairs and double bonds; the molecule should have both these requirements in order to show resonance. Moreover, this effect causes the polarity of a molecule.
There can be interactions between lone electron pairs and pi bonds (double bonds) adjacent to each other. Therefore, the number of resonance structures that a molecule can have depends on the number of lone electron pairs and pi bonds. Then we can determine the actual structure of the molecule by looking at the resonance structures; it is a hybrid structure of all the resonance structures. This hybrid structure has lower energy than all the other resonance structures. Therefore, it is the most stable structure.
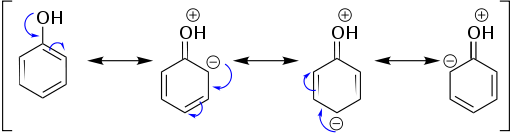
Figure 01: Resonance Structures of Phenol
There are two forms of resonance as a positive resonance effect and negative resonance effect. They describe the delocalization of electrons in positively charged molecules and in negatively charged molecules respectively. As a result, these two forms stabilize the electrical charge of the molecule.
What is Mesomeric Effect?
Mesomeric effect is a theory in chemistry that describes the stabilization of molecules having different substituent groups and functional groups. This occurs mainly because some substituent groups act as electron donors while some of them act as electron withdrawers. The differences between the electronegativity values of the atoms in the substituent group make it either an electron donor or a withdrawer.
Some examples for these groups are as follows;
- Electron donor substituents; –O, -NH2, -F, -Br, etc.
- Electron withdrawing substituents; –NO2, -CN, -C=O, etc.
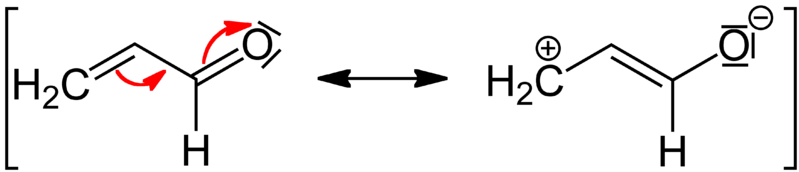
Figure 02: Negative Mesomeric Effect
Moreover, the electron donating substituents cause negative mesomeric effect while electron withdrawing substituents cause a positive mesomeric effect. Apart from that, in conjugated systems, the mesomeric effect moves along the system. It involves the delocalization of pi bond electron pairs. Hence, this stabilizes the molecule.
What is the Difference Between Resonance and Mesomeric Effect?
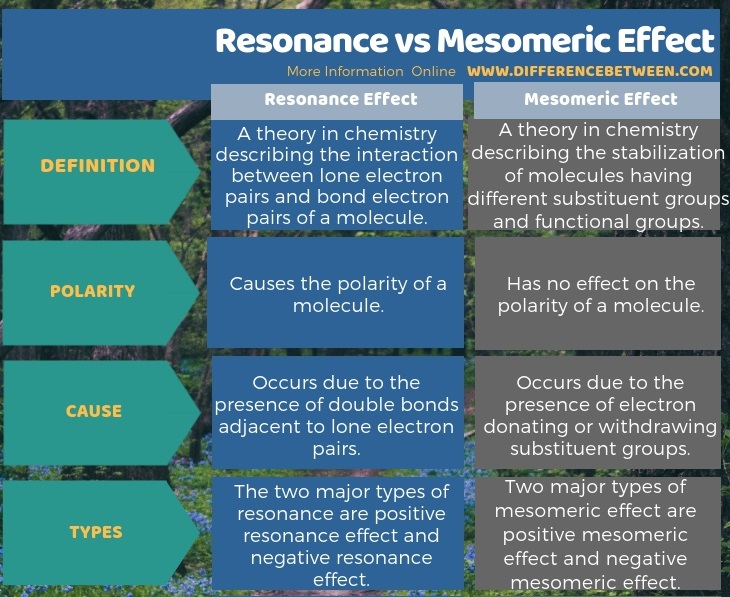
Resonance is a theory in chemistry which describes the interaction between lone electron pairs and bond electron pairs of a molecule whereas Mesomeric effect is a theory in chemistry which describes the stabilization of molecules having different substituent groups and functional groups. This is the fundamental difference between resonance and mesomeric effect. Furthermore, though the resonance has a direct influence on the polarity of a molecule, mesomeric effect has no considerable effect. Moreover, there is also a difference between resonance and mesomeric effect in their cause of occurence. Resonance occurs due to the presence of double bonds adjacent to lone electron pairs while mesomeric effect occurs due to the presence of electron donating or withdrawing substituent groups.
Summary – Resonance vs Mesomeric Effect
Resonance and mesomeric effect are common in complex organic molecules. The key difference between resonance and mesomeric effect is that resonance is a result of interaction between lone electron pairs and bond electron pairs whereas mesomeric effect results due to the presence of substituent groups or functional groups.
Reference:
1. “Resonance.” Wikipedia, Wikimedia Foundation, 9 Sept. 2018. Available here
2. “Mesomeric Effect.” Wikipedia, Wikimedia Foundation, 21 July 2018. Available here
Image Courtesy:
1.”Phenol resonance”By Smallman12q – Own work, (Public Domain) via Commons Wikimedia
2.”Mesomeric effect (–M) V.1″ By Jü – Own work, (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2BxKymp5memLJurc2dZKado6S6pr7InGSenpaasLV7