Difference Between Steel and Graphite Irons
The key difference between steel and graphite irons is that steel has low carbon content whereas graphite irons contain high carbon content. Steel is a metal alloy that has iron, carbon and some other elements mixed with each other while graphite iron is an iron alloy that has graphite along with iron.
Both steel and graphite irons are alloys of iron and differ in carbon content.
CONTENTS
1. Overview and Key Difference
2. What is Steel
3. What are Graphite Irons
4. Side by Side Comparison – Steel vs Graphite Irons in Tabular Form
5. Summary
What is Steel?
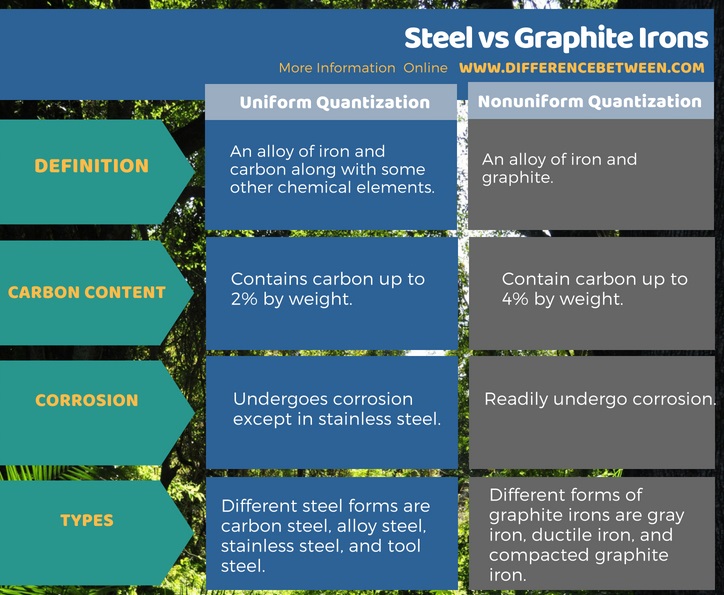
Steel is an alloy of iron and carbon along with some other chemical elements. The carbon content in this alloy ranges up to 2% by weight. The most important properties of this alloy are high tensile strength and low cost. This is the most common material for building infrastructures. In addition, it is useful for the production of tools for constructions as well.
The crystal structure of pure iron has a very little resistance to the iron atoms slipping past one another. Hence, pure iron is very ductile. But steel has carbon and some other components that can act as hardening agents. Thus, the ductility of steel is lower than that of pure iron. The crystal structure of pure iron has dislocations that can move, making the iron ductile, but in steel, the components such as carbon can prevent the movement of these dislocations via entering into the crystal structure of iron.

Figure 01: Chairs Made of Steel
There are 4 different types of steel;
Moreover, steel undergoes corrosion when exposed to air and moisture, except stainless steel. Stainless steel has chromium that gives the property of corrosion resistance by forming a chromium oxide layer on the steel surface when it is exposed to normal air.
What are Graphite Irons?
Graphite iron is an alloy of iron and graphite. There are several types of graphite irons as follows. These types are different from each other according to their chemical and physical properties because this steel has different amounts of carbon in the form of graphite.
- Gray iron– this form has a gray color. The graphite looks flaked. It has a high machinability and wears resistance.
- Ductile iron/ spheroidal graphite iron– graphite occurs in the form of nodules. The ductility toughness is very high.
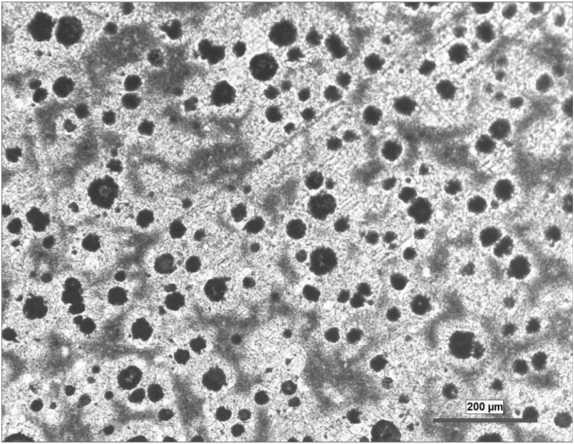
Figure 02: The Microstructure of Ductile Iron
- Compacted graphite iron – this contains graphite as worm-like structures. This graphite occurs as short and very thick particles. The properties of this type are in between gray iron and ductile iron.
What is the Difference Between Steel and Graphite Irons?
Summary – Steel vs Graphite Irons
Steel and graphite irons are forms of iron alloys that contain iron and carbon. The difference between steel and graphite irons is that steel has low carbon content whereas graphite irons contain a high carbon content.
Reference:
1. “Steel.” Wikipedia, Wikimedia Foundation, 12 May 2018. Available here
2. “Compacted Graphite Iron.” Wikipedia, Wikimedia Foundation, 1 May 2018. Available here
Image Courtesy:
1.’816091′ (Public Domain) via pixhere
2.’Ductile Iron’By Michelshock – McGill University, (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2F056cpWWRo7Fus9Gap6GhpJp6qr7Op6po