Difference Between Substitutional and Interstitial Solid Solution
The key difference between substitutional and interstitial solid solution is that the substitutional solid solution involves the substitution of a solvent atom by a solute atom, in its formation. On the contrary, there is no displacement of solvent atoms by solute atoms in the formation of interstitial solid solutions, instead, the solute molecules enter the holes between solvent atoms.
A solid solution is a solid state solution of one or more solutes in the same solvent. There, two or more elements occur in the solid state. We call it a solution rather than a compound because the crystal structure of the solvent remains unchanged with the addition of solutes. In the process of solid solution strengthening, the strength of pure metal can be increased via alloying another element. We can do this by forming a solid solution. Depending on the alloying element, there are two forms of solid solutions as substitutional and interstitial solid solutions.
CONTENTS
1. Overview and Key Difference
2. What is Substitutional Solid Solution
3. What is Interstitial Solid Solution
4. Side by Side Comparison – Substitutional vs Interstitial Solid Solution in Tabular Form
5. Summary
What is Substitutional Solid Solution?
Substitutional solid solutions are solid-state solutions that form when the solute atoms replace the solvent atoms. In order to form this kind of solid solution, the solute atoms should be large enough to replace the solvent atoms in the lattice. The solute atoms incorporate into the lattice substitutionally via replacing the atoms in particular positions of the lattice. Here, some alloying elements have solute atoms which are able to replace solvent atoms only in small amounts while there are some alloying elements which can replace solvent atoms all over the solid solution.
This type of solid solution forms when the solute and solvent atoms have nearly similar atomic sizes. In addition, high temperatures can increase these replacements. Moreover, there are two forms of substitutional solid solutions namely; ordered and disordered solid solutions. An ordered solid solution form when the solute atoms replace the solvent atoms in a preferred site of the solvent lattice. Ex: Cu-Au system. Disordered solid solutions form when the solute atoms randomly replace the solvent atoms in the lattice.
What is Interstitial Solid Solution?
Interstitial solid solutions are solid state solutions that form when solute atoms enter into the holes between solvent atoms of the lattice. There, the solute atoms are small enough to enter into these holes. We call these holes, interstitial sites.
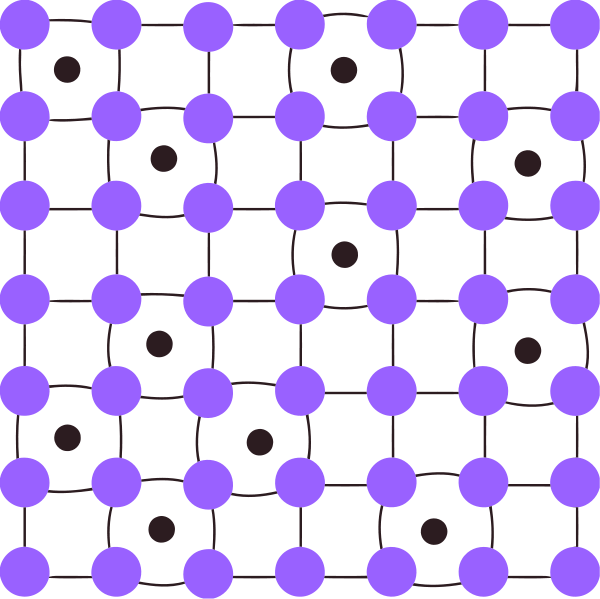
Figure 01: An Interstitial Solid Solution
This process weakens the bonds between solvent atoms. Thus, the lattice deforms. For this to happen the atomic size of solute atoms should be less than 40% of the size of solvent atoms. The solute atoms enter into the lattice interstitially. The only elements that are capable of forming this type of solid solutions are H, Li, Na and B.
What is the Difference Between Substitutional and Interstitial Solid Solution?
Substitutional solid solutions are solid-state solutions that form when the solute atoms replace the solvent atoms. This type of solid solutions forms only if the solute atoms are large enough to replace the solvent atoms in the lattice. Moreover, the atomic size of solutes is nearly similar to the size of solvent atoms. Interstitial solid solutions are solid state solutions that form when solute atoms enter into the holes between solvent atoms of lattice. These solid solutions form only if the solute atoms are small enough to enter the holes of the lattice. In addition, the atomic size of solute atoms should be about 40% of the size of solvent atoms in order to form this type of lattice. This is the main difference between substitutional and interstitial solid solution.
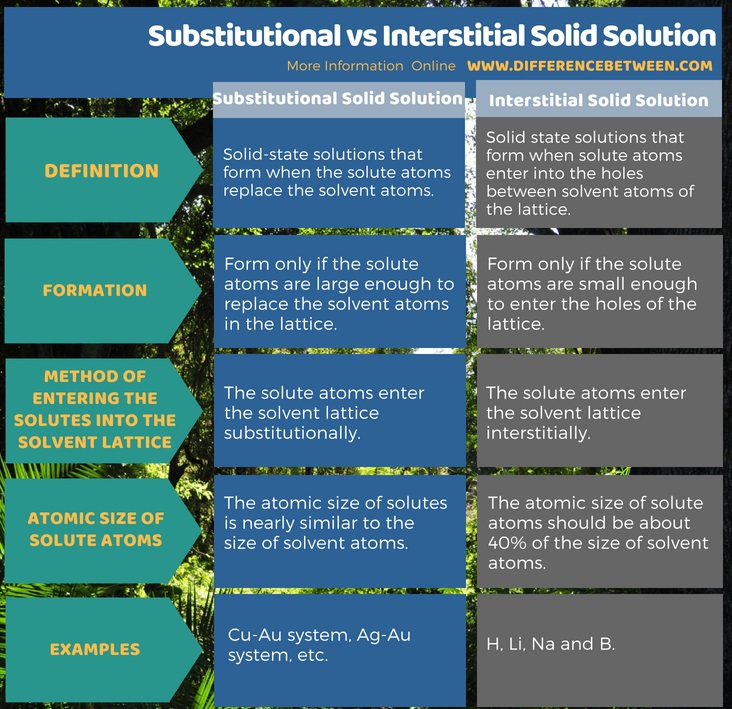
Summary – Substitutional vs Interstitial Solid Solution
Solid solutions are the solid state solutions which have two or more types of elements in the same solid state mixture. There are two forms of solid solutions as substitutional and interstitial solid solutions according to the way it forms. The difference between substitutional and interstitial solid solution is that in the formation of a substitutional solid solution, it involves the substitution of a solvent atom by a solute atom whereas in the formation of interstitial solid solutions, there is no displacement of solvent atoms by solute atoms, instead, the solute molecules enter the holes between solvent atoms.
Reference:
1. “Solid Solution Strengthening.” Wikipedia, Wikimedia Foundation, 19 June 2018. Available here
2. SMALL ARMS FACTORY (MINISTRY OF DEFENSE) Follow. “Solid Solutions.” LinkedIn SlideShare, 18 Nov. 2013. Available here
Image Courtesy:
1.’Interstitial solute’By ISiamrut at English Wikipedia (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26%2F1JuqraGkqsGqu82ao2aZnpl6qrrTnqmsrJmptqK4jKympaGUYsCwuNStoKimXw%3D%3D