Difference Between Tartaric Acid and Citric Acid
The key difference between tartaric acid and citric acid is that the tartaric acid (cream of tartar, C4H6O6) is diprotic whereas the citric acid (C6H8O7) is triprotic. Tartaric acid is commercially available as a white powder and has a very poor water solubility while citric acid is an odorless compound and is available as a solid crystalline compound.
Tartaric acid and citric acid are acidic compounds because their carboxylic groups can release the hydrogen atoms in them to the medium making the medium acidic. Both these compounds are present in plants, most notably in fruits. Tartaric acid is present in grapes whereas citric acid is present in lemons.
CONTENTS
1. Overview and Key Difference
2. What is Tartaric Acid
3. What is Citric Acid
4. Side by Side Comparison – Tartaric Acid vs Citric Acid in Tabular Form
5. Summary
What is Tartaric Acid?
Tartaric acid, commonly known as cream of tartar, is an organic compound having the chemical formula C4H6O6. The IUPAC name of this acid is 2,3-Dihydroxybutanedioic acid. The molar mass of this acid is 150.08 g/mol and it has a very poor water solubility. The compound is available as a white powder and is an irritant in the concentrated form.
Tartaric acid is naturally available in grapes and forms spontaneously during the winemaking process using grapes. Further, it is common in its potassium salt form – potassium bitartrate. Baking powder, a common leavening agent in food production, is a mixture of sodium bicarbonate and potassium bitartrate. Moreover, tartaric acid function as an antioxidant in some food.
Tartaric acid is an alpha-hydroxy-carboxylic acid. This categorization is because of the two carboxylic acid groups in this molecule and both these groups have a hydroxyl group at their alpha carbon position. Further, the molecule is diprotic since it is possible to remove the hydrogen atoms in the two carboxylic groups as protons.
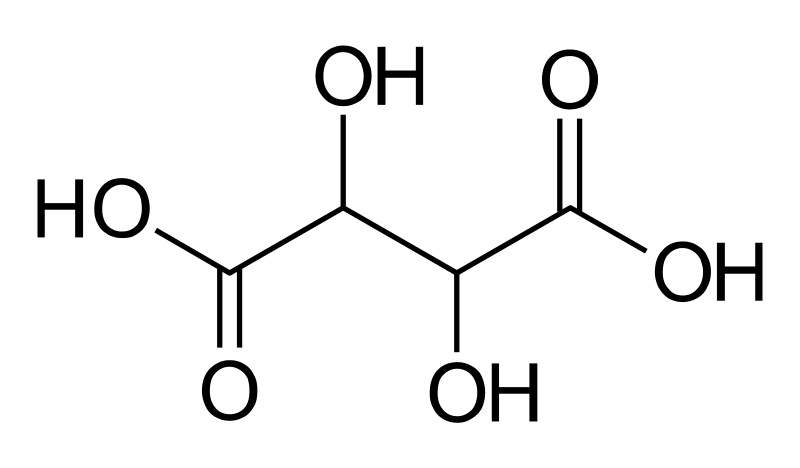
Figure 1: Tartaric Acid Molecule
The naturally occurring tartaric acid molecule is a chiral compound. That means, this molecule has enantiomers; it has L and D enantiomers. The naturally occurring enantiomer is the L-(+)-tartaric acid. These enantiomers are optically active because they can rotate the plane-polarized light.
What is Citric Acid?
Citric acid is an organic compound having the chemical formula C6H8O7. The IUPAC name of this compound is 2-Hydroxypropane-1,2,3-tricarboxylic acid. Its molar mass is 192.12 g/mol and melting point is 156 °C. It is an odorless compound and is available as a solid crystalline compound.
The citric acid molecule has three carboxylic acid group, indicating that it is tribasic or triprotic, but has only one hydroxyl group. The acid is triprotic because the acid molecule can release three protons per molecule (the three carboxylic acid groups can release the hydrogen atoms in them as protons).
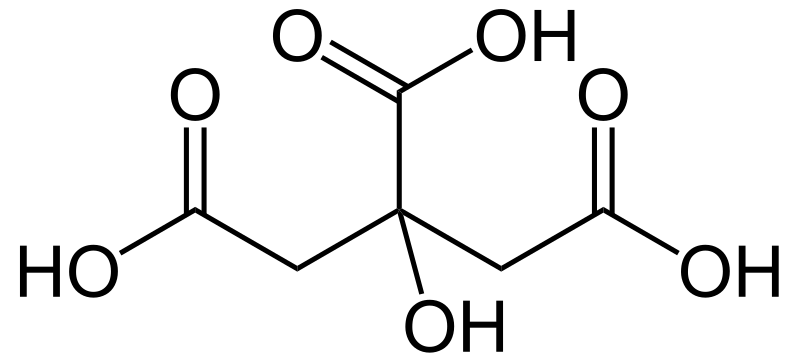
Figure 2: Citric Acid Molecule
Citric acid is naturally available in lemon and other fruits in the Rutaceae family, i.e., citrus fruits. It is a skin and eye irritant. This compound has different applications, such as food additives, drink, chelating agent, ingredient in certain cosmetics, etc.
What is the Difference Between Tartaric Acid and Citric Acid?
Tartaric Acid vs Citric Acid | |
| Tartaric acid is an organic compound having the chemical formula C4H6O6. | Citric acid is an organic compound having the chemical formula C6H8O7. |
| IUPAC Name | |
| 2,3-Dihydroxybutanedioic acid | 2-Hydroxypropane-1,2,3-tricarboxylic acid |
| Molar Mass | |
| 150.08 g/mol | 192.12 g/mol |
| Melting Point | |
| 206 °C (in racemic mixture of D and L enantiomers) | 153 °C |
| Boiling Point | |
| 275 °C | 310 °C |
| Number of Carboxylic Acid Groups | |
| Has two carboxylic acid groups | Has three carboxylic acid groups |
| Presence of Enantiomers | |
| Two enantiomer forms: L-tartaric acid and D-tartaric acid | No enantiomers |
| Presence of Hydroxyl Group | |
| Has two hydroxyl groups | Has one hydroxyl group |
| Natural Source | |
| Naturally available in fruits such as grapes | Available in citrus fruit naturally |
| Commerical Product | |
| Sold as baking soda | Sold as a crystalline white solid |
| Applications | |
| Used in the pharmaceutical industry and as a chelating agent for calcium and magnesium | Used as an ingredient in food and beverages, as a chelating agent, in the production of pharmaceuticals and cosmetics, etc. |
Summary – Tartaric Acid vs Citric Acid
The key difference between tartaric acid and citric acid is that the tartaric acid is diprotic whereas citric acid is triprotic. That means, the tartaric acid molecule has two hydrogen atoms to release as protons while citric acid molecule has three hydrogen atoms to release as protons. Both these acidic compounds are commonly available in plants, notably in fruits; but, grapes is the common source of tartaric acid while citrus fruits are the common source of citric acid.
Reference:
1. “Tartaric Acid.” Wikipedia, Wikimedia Foundation, 22 Apr. 2018, Available here.
2. “Citric Acid.” Wikipedia, Wikimedia Foundation, 17 Apr. 2018, Available here.
3. Brown, William H. “Tartaric Acid.” Encyclopædia Britannica, Inc., 17 Apr. 2016, Available here.
Image Courtesy:
1. “Tartaric acid” By JaGa – self-made using BKChem and Inkscape (CC BY-SA 3.0) via Commons Wikimedia
2. “Zitronensäure – Citric acid” By NEUROtiker – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27AwKurmqqZmHqir8idZJqmlGKwqsDRoppmmZOesXA%3D