Difference Between Trypsin and Chymotrypsin
Key Difference – Trypsin vs Chymotrypsin
Protein digestion is a very important process in the overall digestion procedure in living organisms. Complex proteins are digested into its monomers of amino acids and are absorbed via the small intestines. Proteins are essential as they serve a major functional and a structural role in an organism. Protein digestion takes place via protein digesting enzymes which include trypsin, chymotrypsin, peptidases, and proteases. Trypsin is a protein-digesting enzyme which cleaves the peptide bond at the basic amino acids which include lysine and arginine. Chymotrypsin is also a protein-digesting enzyme which cleaves the peptide bond at aromatic amino acids such as phenylalanine, tryptophan, and tyrosine. The key difference between trypsin and chymotrypsin is the position of the amino acid in which it cleaves in the protein. Trypsin cleaves at basic amino acid positions whereas chymotrypsin cleaves at aromatic amino acid positions.
CONTENTS
1. Overview and Key Difference
2. What is Trypsin
3. What is Chymotrypsin
4. Similarities Between Trypsin and Chymotrypsin
5. Side by Side Comparison – Trypsin vs Chymotrypsin in Tabular Form
6. Summary
What is Trypsin?
Trypsin is a 23.3 kDa protein that belongs to the family of serine proteases and its main substrates are basic amino acids. These basic amino acids include arginine and lysine. Trypsin was discovered in 1876 by Kuhne. Trypsin is a globular protein and exists in its inactive form which is trypsinogen – zymogen. The mechanism of action of trypsin is based upon the serine protease activity.
Trypsin cleaves at the C terminal end of the basic amino acids. This is a hydrolysis reaction and takes place at a pH – 8.0 in the small intestines. The activation of trypsinogen takes place through the removal of the terminal hexapeptide, and it produces the active form; trypsin. Active trypsin is of two main types; α – trypsin and β-trypsin. They differ in their thermal stability and their structure. The active site of trypsin contains Histidine (H63), Aspartic acid (D107) and Serine (S200).
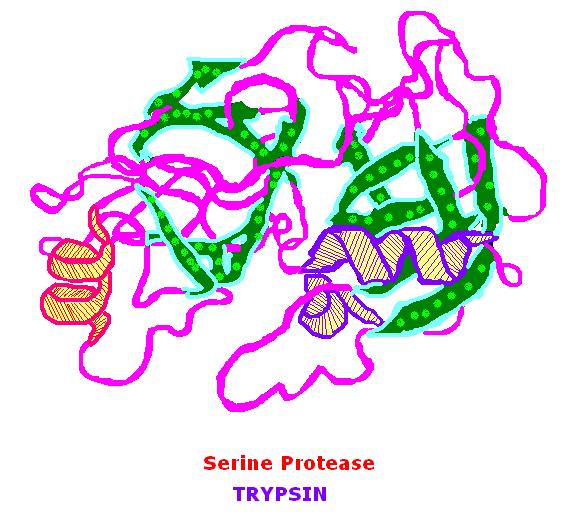
Figure 01: Trypsin
The enzymatic action of trypsin is inhibited by DFP, aprotinin, Ag+, Benzamidine, and EDTA. The applications of trypsin include dissociation of tissue, trypsinization in animal cell culture, tryptic mapping, in vitro protein studies, fingerprinting and in tissue culture applications.
What is Chymotrypsin?
Chymotrypsin has a molecular weight of 25.6 kDa and belongs to the serine protease family, and it is an endopeptidase. Chymotrypsin exists in its inactive form which is chymotrypsinogen. Chymotrypsin was discovered in the year 1900s. Chymotrypsin hydrolyzes the peptide bonds at the aromatic amino acids. These aromatic substrates include tyrosine, phenylalanine, and tryptophan. The substrates of this enzyme are mainly in the L-isomers and readily act upon the amides and esters of amino acids. The optimum pH in which chymotrypsin acts is 7.8 – 8.0. There are two main forms of chymotrypsin such as chymotrypsin A and chymotrypsin B and they slightly differ in there structural and proteolytic characteristics. The active site of chymotrypsin contains a catalytic triad and is composed of Histidine (H57), Aspartic acid (D102) and Serine (S195).
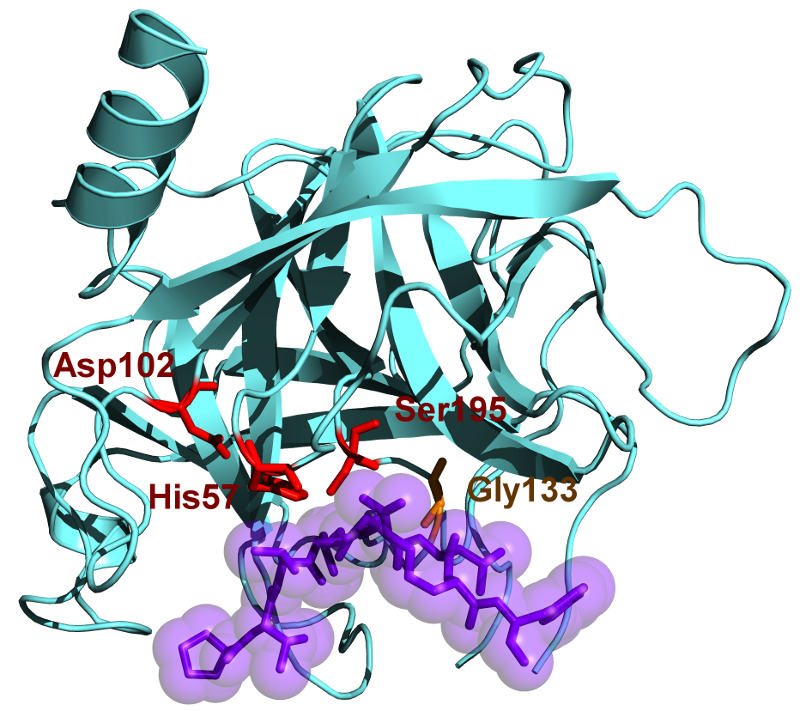
Figure 02: Chymotrypsin
The activators of chymotrypsin are Cetyltrimethylammonium bromide, Dodecyltrimethylammonium bromide, Hexadecyltrimethylammonium bromide and Tetrabutylammonium bromide. The inhibitors of chymotrypsin are peptidyl aldehydes, boronic acids, and coumarin derivatives. Chymotrypsin is commercially used in peptide synthesis, peptide mapping and peptide fingerprinting.
What are the Similarities Between Trypsin and Chymotrypsin?
- Both enzymes are serine proteases.
- Both enzymes cleave peptide bonds.
- Both enzymes act in the small intestine.
- Both enzymes exist in its inactive form as zymogens.
- Both enzymes are composed of a catalytic triad containing histidine, aspartic acid, and serine in its active site.
- Both enzymes were initially discovered and extracted from cattle.
- Production of both enzymes is done through recombinant DNA techniques presently.
- Both enzymes act on an optimal basic pH.
- Both enzymes are used in vitro in different industries.
What is the Difference Between Trypsin and Chymotrypsin?
Trypsin vs Chymotrypsin | |
| Trypsin is a protein-digesting enzyme which will cleave the peptide bond at the basic amino acids such as lysine and arginine. | Chymotrypsin which is also a protein-digesting enzyme cleaves the peptide bond at aromatic amino acids such as phenylalanine, tryptophan, and tyrosine. |
| Molecular Weight | |
| The molecular weight of trypsin is 23.3 k Da. | The molecular weight of chymotrypsin is 25.6 k Da. |
| Substrates | |
| Complex proteins are digested into its monomers of amino acids and are absorbed via the small intestines. | Aromatic amino acids substrates such as tyrosine, tryptophan, and phenylalanine act on chymotrypsin. |
| Zymogen Form of the Enzyme | |
| Trypsinogen is the inactive form of trypsin. | Chymotrypsinogen is the inactive form of chymotrypsin. |
| Activators | |
| Lanthanides are activators of trypsin. | Cetyltrimethylammonium bromide, Dodecyltrimethylammonium bromide, Hexadecyltrimethylammonium bromide and Tetrabutylammonium bromide are activators of chymotrypsin. |
Inhibitors | |
| DFP, aprotinin, Ag+, Benzamidine, and EDTA are inhibitors of trypsin. | Peptidyl aldehydes, boronic acids, and coumarin derivatives are inhibitors of chymotrypsin. |
Summary – Trypsin vs Chymotrypsin
Peptidases or proteolytic enzymes cleave proteins via the hydrolysis of the peptide bond. Trypsin cleaves the peptide bond at basic amino acids whereas chymotrypsin cleaves the peptide bond at aromatic amino acid residues. Both enzymes are serine peptidases and act in the small intestine in a basic pH environment. At present, much research is involved in producing trypsin and chymotrypsin using recombinant DNA technology by using different bacterial and fungal species as these enzymes possess a high industrial value. This is the difference between trypsin and chymotrypsin.
Download the PDF Version of Trypsin vs Chymotrypsin
You can download PDF version of this article and use it for offline purposes as per citation note. Please download PDF version here Difference Between Trypsin and Chymotrypsin
Reference:
1.“Chymotrypsin.” Chymotrypsin – Worthington Enzyme Manual. Available here
2.“Trypsin.” Trypsin – Worthington Enzyme Manual. Available here
Image Courtesy:
1.’Serine protease’By Tinastella at English Wikibooks (Public Domain) via Commons Wikimedia
2.’Chymotrypsin 4cha’By Yikrazuul – Own work, (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27A0bKnrKGeYq6vsIyvqmabmK66sMDRsqesoZ5k