Difference Between Vinylic Halides and Aryl Halides
The key difference between vinylic halides and aryl halides is that vinylic halides are named according to the carbon atom to which the halide atom is bonded whereas aryl halides are named depending on the presence of a cyclic structure to which the halide atom is attached.
Vinylic halides and aryl halides are organic chemical compounds in which halide atoms (group 7 chemical elements) are bonded directly to carbon atoms. We can categorize these compounds depending on the carbon atom to which the halide atom is attached.
CONTENTS
1. Overview and Key Difference
2. What are Vinylic Halides
3. What are Aryl Halides
4. Side by Side Comparison – Vinylic Halides vs Aryl Halides in Tabular Form
5. Summary
What are Vinylic Halides?
Vinylic halides are organic compounds with a halide atom bonded to one of the two carbon atoms in the double bond present in that molecule. Therefore, these are alkenes containing a chlorine atom at the double bond. In other words, the carbon atom containing the halide atom has sp2 hybridization, and its geometry around the carbon atom is trigonal planar. These carbon atoms in the double bond are called vinylic carbons. The electron density around these carbon centres are higher; however, the carbon atom containing the halide atom has more electron density than the other carbon atom because the chlorine atom is an electron-rich species.
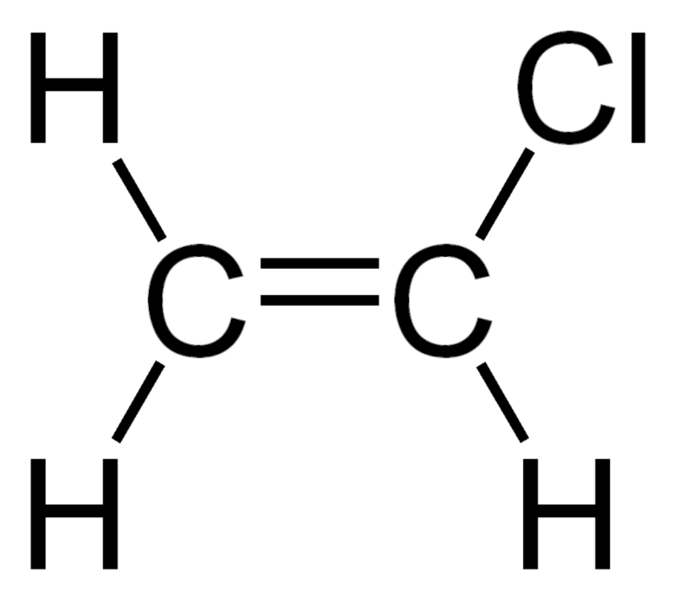
Figure 01: Vinyl Chloride Structure
Vinyl chloride is a common compound in this class of organic compounds. This compound exists as a colourless gas at room temperature, and it has a pleasant odour as well. Furthermore, it is important as the monomer for the production of polyvinyl chloride polymer. Therefore, vinyl chloride is a chemical intermediate, rather than a final product. The polymer product of vinyl chloride (polyvinyl chloride) is stable, storable and non-toxic.
What are Aryl Halides?
An aryl halide is an organic compound having a halogen atom attached directly to an sp2 hybridized carbon in an aromatic ring. Therefore, we can name this compound as an unsaturated structure due to the presence of double bonds in the aromatic ring. Aryl halides also show dipole-dipole interactions. The carbon-halogen bond is stronger than that of alkyl halides due to the presence of ring electrons. This happens because the aromatic ring gives electrons to the carbon atom, reducing the positive charge. Aryl halides can undergo electrophilic substitution and can get alkyl groups attached to the ortho, para or meta positions of the aromatic ring. One or two halogens can also get attached to the aromatic ring. That is also in the ortho, para or meta positions.
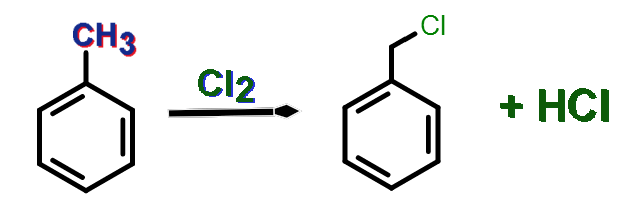
Figure 02: Formation of Benzyl Chloride
Alkyl halides are the other major group of halide compounds. We can use a simple chemical test to distinguish between aryl and alkyl halides. Here, we can use a chemical test. First, NaOH should be added, followed by heating. Then the mixture is cooled, and HNO3 id is added, followed by the addition of AgNO3. Alkyl halide will give a white precipitate whereas aryl halide will not. That is because aryl halides do not undergo nucleophilic substitution, unlike alkyl halides. The reason for not undergoing nucleophilic substitution is that the electron cloud of the aromatic ring causes repulsion of the nucleophile.
What is the Difference Between Vinylic Halides and Aryl Halides?
We can categorize halide-organic compounds depending on the carbon atom to which the halide atom is attached. The key difference between vinylic halides and aryl halides is that vinylic halides are named according to the carbon atom to which the halide atom is bonded whereas aryl halides are named depending on the presence of a cyclic structure to which the halide atom is attached.
Below infographic tabulates more differences between vinylic halides and aryl halides.
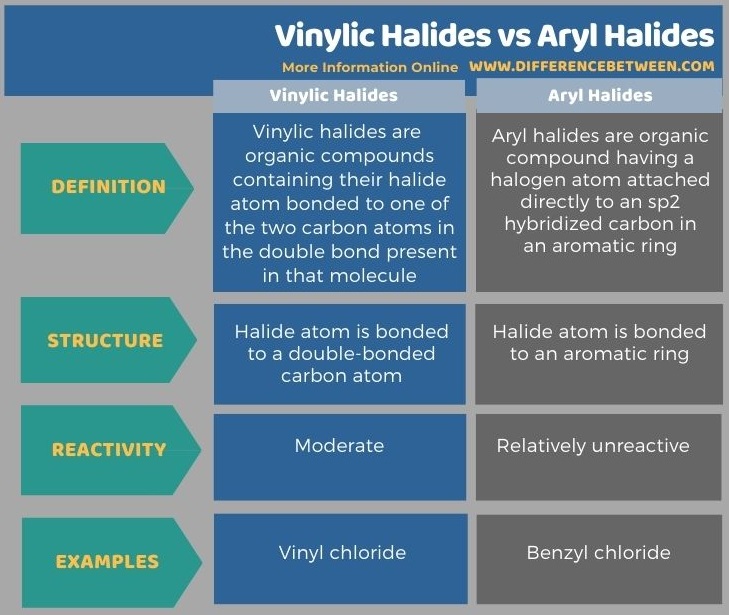
Summary – Vinylic Halides vs Aryl Halides
Halide-containing organic compounds can be classified mainly into two groups as alkyl and aryl halides. Vinylic halides come under the group of alkyl halides. Vinylic halides are organic compounds containing their halide atom bonded to one of the two carbon atoms in the double bond present in that molecule while aryl halides are organic compound having a halogen atom attached directly to an sp2 hybridized carbon in an aromatic ring. So, this is the key difference between vinylic halides and aryl halides.
Reference:
1. “Reactions of Aryl Halides.” Organic Chemistry II – Cliffnotes, Available here.
Image Courtesy:
1. “Vinilxlorid” By Kəmalə baxa – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Benzyl chloride synthesis” By Claudio Pistilli – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27CyKewpaGTYrWiuMidnKxlkaOxbq3RsqNmoJGhtqWx0mg%3D