Difference Between Volatile and Nonvolatile Acids
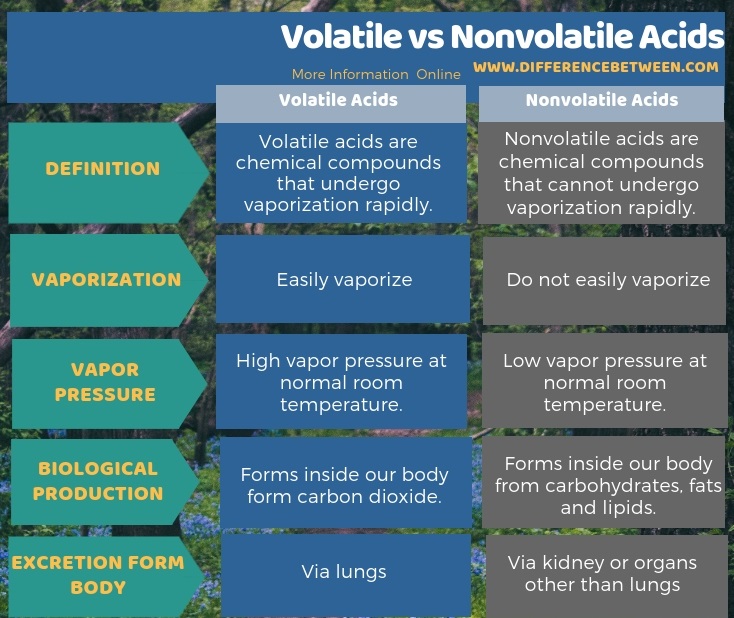
The key difference between volatile and nonvolatile acids is that the volatile acids easily vaporize whereas the nonvolatile acids do not easily vaporize.
Volatility is the tendency of a substance to vaporize. Therefore, volatile substances go into the vapor phase easier than the nonvolatile substances. However, this vaporization may take place with or without heating. The reason for the high volatility is having a high vapor pressure at normal room temperature.
CONTENTS
1. Overview and Key Difference
2. What are Volatile Acids
3. What are Nonvolatile Acids
4. Side by Side Comparison – Volatile vs Nonvolatile Acids in Tabular Form
5. Summary
What are Volatile Acids?
Volatile acids are chemical compounds that undergo vaporization rapidly. This rapid vaporization is a result of having a high vapor pressure at normal room temperature. Therefore, volatile acids can undergo vaporization without heating or any other external force.
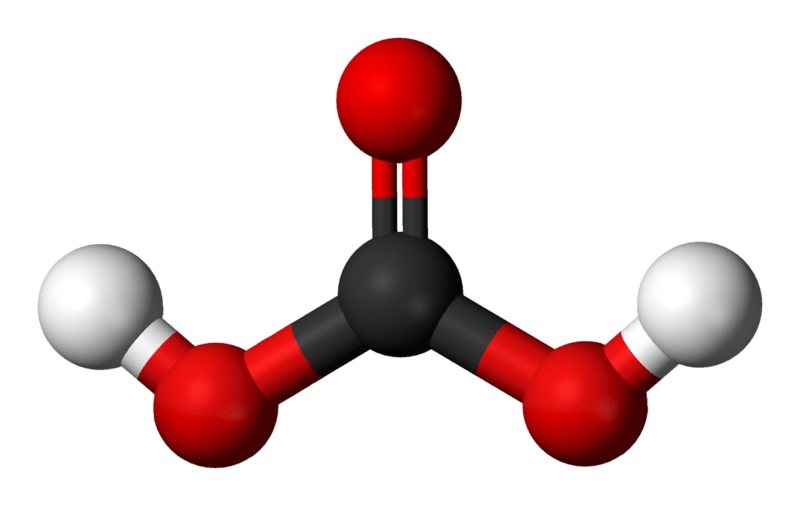
Figure 01: The Chemical Structure of Carbonic Acid
Furthermore, the term volatile acids mainly refer to organic acids that form inside our body due to digestion, diseases or metabolism and also these acids may exist in grape juice, musts and wine. Particularly, carbonic acid is a volatile acid that forms inside our body form carbon dioxide. Moreover, the excretion of this acid is via lungs.
What are Nonvolatile Acids?
Nonvolatile acids are chemical compounds that cannot undergo vaporization rapidly. That is mainly because the vapor pressure of the acid at normal room temperature is not high enough to vaporize easily. Therefore, we can name them as fixed acids or metabolic acids because, mainly, our body produces these acids from sources other than carbon dioxide. i.e., the incomplete metabolism of carbohydrates, fats and proteins produce these acids. Except carbonic acid, most of the acids that our body produces are nonvolatile. Also, the excretion of these acids is via the kidney.
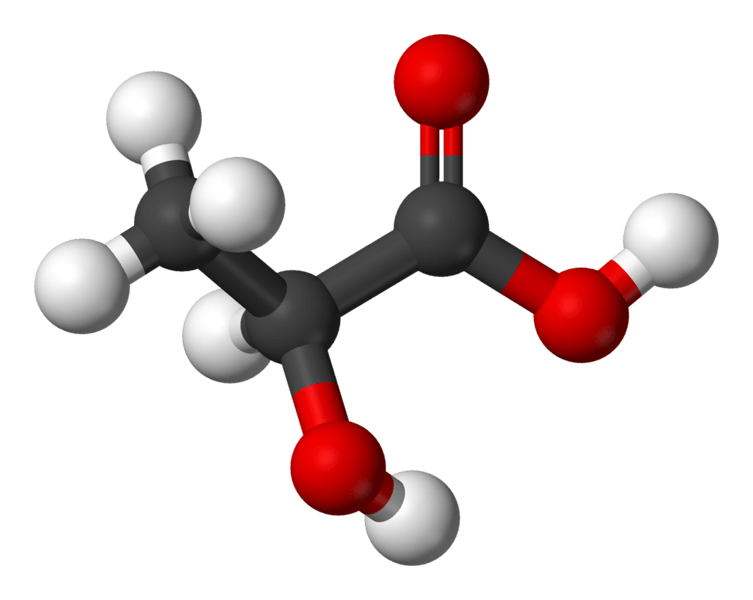
Figure 02: Lactic Acid – a Nonvolatile Acid that is produced inside our Body
The reactions that can cause the production of Nonvolatile acids are as follows:
- Oxidation of sulfur-containing amino acids:
E.g.: Cysteine → urea + CO2 + H2SO4
- Metabolism of phosphorous containing compounds:
- Cationic amino acid oxidation:
Eg: Arginine → urea + CO2 + H2O + H+
- Incomplete metabolism of carbohydrate, fat and lipids.
What is the Difference Between Volatile and Nonvolatile Acids?
Volatile acids are chemical compounds that undergo vaporization rapidly whereas nonvolatile acids are chemical compounds that cannot undergo vaporization rapidly. Thus, this is the key difference between volatile and nonvolatile acids. This difference arises due to the vapor pressures of each acid. Therefore, this gives rise to another difference between volatile and nonvolatile acids. i.e., the vapor pressure of volatile acids is very high at normal room temperature while the vapor pressure of nonvolatile acids is low comparatively.
Furthermore, when considering the volatile and nonvolatile acids that our body produces, the major volatile acid is the carbonic acid that excretes via lungs whereas the nonvolatile acids include sulfuric acid and lactic acid, which excrete via kidney and organs other than lungs. That is mainly because the volatile acids can excrete from the body via ventilation while the nonvolatile acids cannot. Hence, this also contributes to another difference between volatile and nonvolatile acids.
The below infographic shows the difference between volatile and nonvolatile acids in tabular form.
Summary – Volatile vs Nonvolatile Acids
Volatile and nonvolatile acids are chemical compounds that we name according to the ability to vaporize quickly. Therefore, the key difference between volatile and nonvolatile acids is that volatile acids easily vaporize whereas nonvolatile acids do not easily vaporize.
Reference:
1. Volatile Acidity. Available here
2. “Nonvolatile Acid.” Wikipedia, Wikimedia Foundation, 5 Jan. 2018. Available here
Image Courtesy:
1.”Carbonic-acid-3D-balls”By Jynto and Ben Mills (Public Domain) via Commons Wikimedia
2.”Lactic-acid-3D-balls” (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27CzqWYraGcmnqiusNmpaimpqS5osDIpZxmmZOesbR7