Difference Between Volumetric and Gravimetric Analysis
The key difference between volumetric and gravimetric analysis is that the volumetric analysis measures the quantity of an analyte using volume whereas the gravimetric analysis measures the quantity of an analyte using weight.
In an analysis, we measure the amount of an unknown compound with the use of a known amount of a known compound. We can take this amount as a volume or as a weight. If it is volume, we call it “volumetric analysis” or “titrimetric analysis”. If it is weight, we call it “gravimetric analysis”. Both are quantitative analytical techniques because these techniques can measure the amount of a sample.
CONTENTS
1. Overview and Key Difference
2. What is Volumetric Analysis
3. What is Gravimetric Analysis
4. Side by Side Comparison – Volumetric vs Gravimetric Analysis in Tabular Form
5. Summary
What is Volumetric Analysis?
Volumetric analysis is a type of quantitative analysis in which we can measure the amount of an unknown compound using its volume. We can use titrations for this purpose. Therefore, we call this analysis a “titrimetric analysis”. In a titration, we use a second solution or reagent for the determination of the volume of the unknown compound present in a sample. By determining the volume of the unknown, we can determine the concentration of that compound in the sample.
Volumetric Analysis for a Titration
For a titration, there are several components we need in the experimental system. Those components include a burette, burette holder, a beaker or an Erlenmeyer flask and pipettes. Typically, we fill the reagent (having a known concentration) into the burette and should take the sample (containing the unknown compound) into the beaker (a known volume). In addition, we should use indicators for the determination of the endpoint of the titration. Moreover, it is important to choose the correct indicator for a particular titration according to the pH range in which we do the titration. Ex: the indicator phenolphthalein works at the pH range of 8.3-10.0. The indicator gives a colour change at the endpoint. Ex: the colour of phenolphthalein at pH 8.3 is colorless, and at pH 10.0, it shows a pale pink colour.

Figure 01: An Acid-Base Titration
Moreover, the second reagent that we are filling into the burette should have a considerable reaction in order to give an endpoint (unless it does not give an endpoint or a change of the colour of the indicator). What we measure is the volume of the reagent (in the burette) which reacts with the compound in the sample. We can use stoichiometric relationships to determine the moles of the unknown present in the sample using the following equation.
C1V1 = C2V2
Here C1 is the concentration of the reagent in the burette, V1 is the volume of the reagent which reacts with the sample, C2 is the unknown concentration of the sample, and V2 is the volume of the sample which we took into the beaker for the analysis.
What is Gravimetric Analysis?
Gravimetric analysis is a type of quantitative analysis in which we can determine the weight of an unknown compound in a sample. This method involves precipitation reactions for the separation of the desired compound from a sample. A precipitation reaction can convert a dissolved compound into a precipitate which we can weigh. If the sample is a mixture of several solids, we can first dissolve the sample in a suitable solvent and then we can add a suitable reagent which can precipitate the compound we need. We call it a precipitating agent. Eventually, we can separate the precipitate via filtration and weigh.
Most importantly, the precipitating agent should precipitate only the required compound. In addition, the filtration of the should wash off all the other constituents other than the required compound. For the removal of unwanted constituents that still present on the precipitate, we can wash the precipitate using water or any other solvent that does not dissolve the precipitate. Then we can dry the precipitate and weigh.
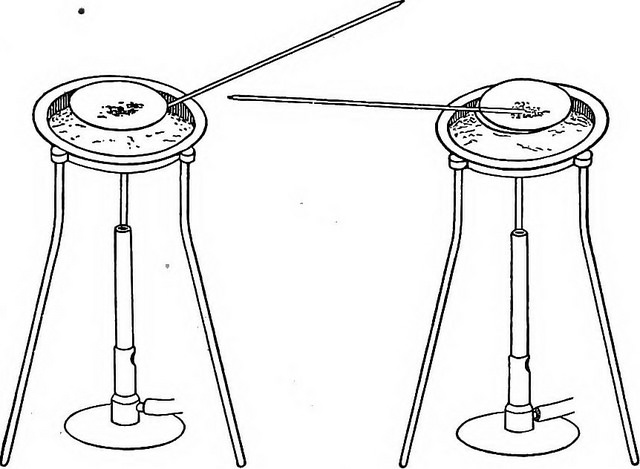
Figure 02: Evaporation of Volatile Compounds in order to Isolate the Precipitate
Other than precipitation, we can analyze a compound by evaporating the volatile components in the sample at an appropriate temperature. We can do this by either heating or chemically decomposing the sample. The volatilisation may be either direct or indirect. Ignition is an example of a direct method. An example of an indirect method is the measuring of loss of the water content from the sample during the heat treatment.
What is the Difference Between Volumetric and Gravimetric Analysis?
Volumetric analysis is a type of quantitative analysis in which we can measure the amount of an unknown compound using its volume. It measures the volume of the desired compound in the units of volume such as L (litres), mL, m3 or dm3. Gravimetric analysis is a type of quantitative analysis in which we can determine the weight of an unknown compound in a sample. It measures the mass of the desired compound in the units of mas such as mg, g and kg. This is the main difference between volumetric and gravimetric analysis.
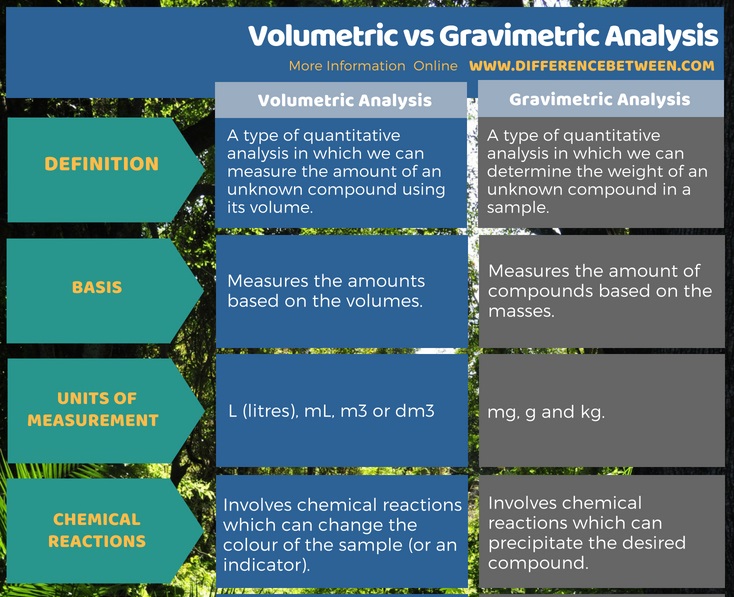
Summary – Volumetric vs Gravimetric Analysis
We can determine the amount of a compound that is present in a given sample using either volumetric analysis or gravimetric analysis. The difference between volumetric and gravimetric analysis is that the volumetric analysis (or titrimetric analysis) measures the quantity of an analyte using volume whereas the gravimetric analysis measures the quantity of an analyte using the weight.
Reference:
1. Britannica, The Editors of Encyclopaedia. “Volumetric Analysis.” Encyclopædia Britannica. Encyclopædia Britannica, Inc., 24 June 2014. Available here
2. Britannica, The Editors of Encyclopaedia. “Gravimetric Analysis.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 17 Apr. 2018. Available here
Image Courtesy:
1.’Phenolphthalein in Flask’By 384 – Own work, (CC BY-SA 4.0) via Commons Wikimedia
2.’14765613865′ by Internet Archive Book Images via Flickr
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau27CzqWspp2kp7akecCnm2afopbDqrnEramim12Wu6K42KygrGc%3D