What is the Difference Between Fatty Alcohol Fatty Acid and Fatty Ester
The key difference between fatty alcohol fatty acid and fatty ester is that fatty alcohols contain a terminal alcohol functional group and fatty acids contain a terminal carboxylic acid functional group, whereas fatty esters contain an ester functional group anywhere in the molecule.
Fatty alcohol is a usually high molecular weight, straight-chain primary alcohol derived from fats and oils. Fatty acids are carboxylic acids containing long aliphatic carbon chains that are either saturated or unsaturated. Moreover, fatty esters or fatty acid esters are a type of esters created from the combination of a fatty acid with an alcohol.
CONTENTS
1. Overview and Key Difference
2. What is a Fatty Alcohol
3. What is a Fatty Acid
4. What is a Fatty Ester
5. Fatty Alcohol vs Fatty Acid vs Fatty Ester in Tabular Form
6. Summary – Fatty Alcohol vs Fatty Acid vs Fatty Ester
What is a Fatty Alcohol?
Fatty alcohol is a usually high molecular weight, straight-chain primary alcohol derived from fats and oils. The chain length of the fatty alcohol varies with the source. Some of the commercially important fatty alcohols include lauryl, stearyl, and oleyl alcohols. These fatty alcohols are colorless oily liquids or waxy solids that might appear in yellow color if there are impurities. Typically, the number of carbon atoms in the fatty alcohol is an even number. Generally, it has a single alcohol group attached to the terminal carbon atom.
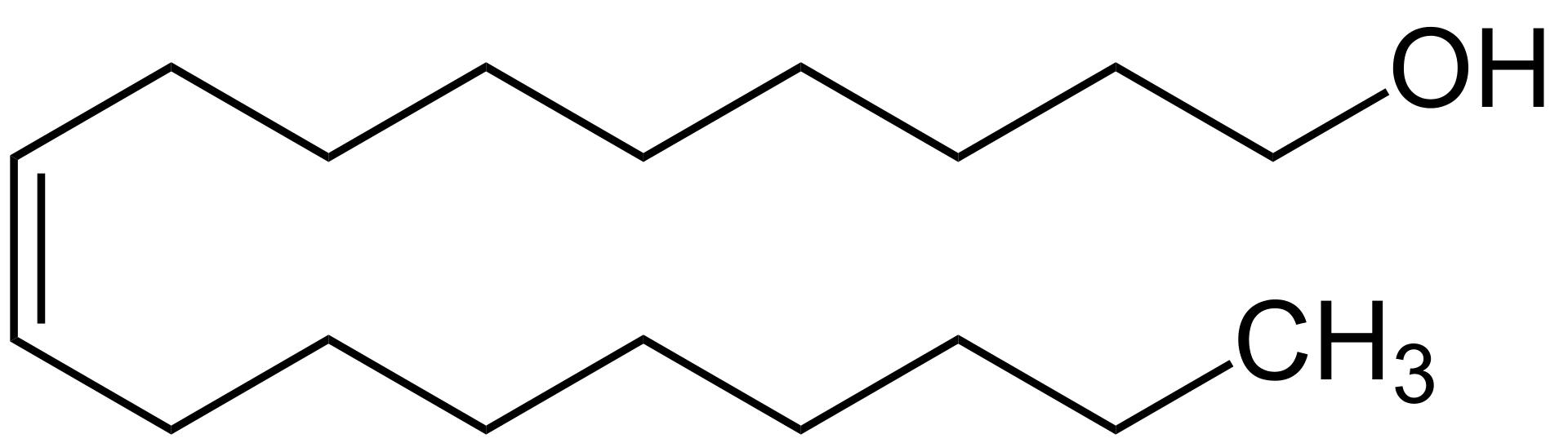
Figure 01: The Chemical Structure of Fatty Alcohol
Some fatty alcohols are unsaturated, while others are branched structures. These fatty alcohols are very important in the industry. Similar to fatty acids, fatty alcohols are often referred to generically with the number of carbon atoms in the molecule, e.g. C-12 alcohol has 12 carbon atoms.
What is a Fatty Acid?
Fatty acids are carboxylic acids containing long aliphatic carbon chains that are either saturated or unsaturated. This means the aliphatic chain may or may not contain double bonds between the carbon atoms. Cis and trans fatty acids are two forms of unsaturated fatty acids.
Cis fatty acids are carboxylic acids containing long aliphatic carbon chains having the two hydrogen atoms attached to a double bond on the same side of the carbon chain. We name this the “cis configuration of unsaturated fatty acids.”
Since hydrogen atoms are on the same side of the carbon chain, it causes the chain to bend. This restricts the conformational freedom of the fatty acid. If there are many double bonds in the chain, it lessens the flexibility of the chain. Moreover, if there are more cis configurations along the carbon chain, it makes the chain quite curved in its most accessible conformations. Examples include cis-oleic acid and cis-linoleic acid.
Trans fatty acids are carboxylic acids containing long aliphatic carbon chains having the two hydrogen atoms attached to a double bond on the opposite sides of the carbon chain. This does not cause the carbon chain to bend much. Moreover, their shape is similar to straight saturated fatty acids. Trans fatty acids are not that much common in nature as cis configuration. It forms mainly as a result of industrial production. For example, hydrogenation reactions may cause the formation of trans fatty acids.
What is a Fatty Ester?
Fatty esters or fatty acid esters are a type of ester that forms from the combination of a fatty acid with an alcohol. If the alcohol component used here is glycerol, then the fatty acid esters that are formed can be named as monoglyceride, diglyceride, or triglyceride according to the structure. Chemically, dietary fats are triglycerides.
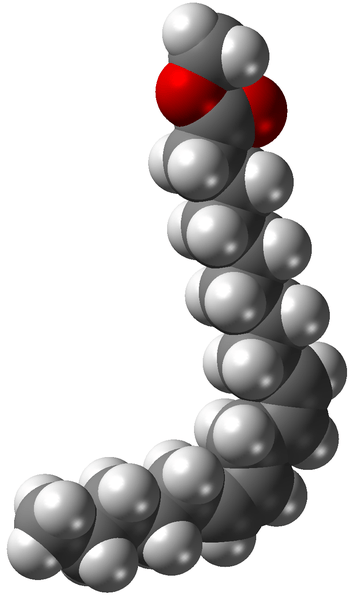
Figure 02: Fatty Ester Molecule
Generally, fatty esters are colorless compounds; however, sometimes, they appear yellowish or brownish if the sample is degraded. Furthermore, triglycerides occur as powders, flakes, coarse powders, and granular or waxy lumps, oils, or liquids. These substances are almost odorless.
What is the Difference Between Fatty Alcohol Fatty Acid and Fatty Ester?
Fatty alcohol is a usually high molecular weight, straight-chain primary alcohol derived from fats and oils. Fatty acids are carboxylic acids containing long aliphatic carbon chains that are either saturated or unsaturated, while fatty esters or fatty acid esters are a type of ester that comes from the combination of a fatty acid with an alcohol. The key difference between fatty alcohol fatty acid and fatty ester is that fatty alcohols contain a terminal alcohol functional group and fatty acids contain a terminal carboxylic acid functional group, whereas fatty esters contain the ester functional group anywhere in the molecule.
The below infographic presents the differences between fatty alcohol fatty acid and fatty ester in tabular form for side by side comparison.
Summary – Fatty Alcohol vs Fatty Acid vs Fatty Ester
Fatty alcohols, fatty acids, and fatty esters are important lipid compounds. The key difference between fatty alcohol fatty acid and fatty ester is that fatty alcohols contain a terminal alcohol functional group and fatty acids contain a terminal carboxylic acid functional group, whereas fatty esters contain the ester functional group anywhere in the molecule.
Reference:
1. “Fatty Alcohol.” An Overview | ScienceDirect Topics.
Image Courtesy:
1. “Oleyl alcohol Structural Formula V1” By Jü – Own work (Public Domain) via Commons Wikimedia
2. “Methyl Linoleate” By E8 (E8) – self-made with Accelrys DS Visualizer (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoyfmK2sqWKura%2FOoaalZZaWwbXFjJqaopxdlrulecWaq62xXZrAtbHRaA%3D%3D