What is the Difference Between Formaldehyde and Acetaldehyde
The key difference between formaldehyde and acetaldehyde is that formaldehyde contains a hydrogen atom bonded to the aldehyde functional group, whereas acetaldehyde contains a methyl group attached to the aldehyde functional group.
Formaldehyde and acetaldehyde are organic compounds we can categorize as aldehyde compounds. Both these are usually colorless gases at room temperature. But formaldehyde can be found in a liquid state on a commercial scale. Formaldehyde is the simplest aldehyde having the chemical formula CH2O.
CONTENTS
1. Overview and Key Difference
2. What is Formaldehyde
3. What is Acetaldehyde
4. Formaldehyde vs Acetaldehyde in Tabular Form
5. Summary – Formaldehyde vs Acetaldehyde
What is Formaldehyde?
Formaldehyde is the simplest aldehyde having the chemical formula CH2O. The IUPAC name of this compound is Methanal. The molar mass of formaldehyde is 30 g/mol, and at room temperature and pressure, it exists as a colourless gas with a pungent, irritating odour.
Moreover, the melting point of formaldehyde is −92 °C, while the boiling point is −19 °C. This substance contains a carbon atom, two hydrogen atoms, and an oxygen atom bonded to each other via covalent chemical bonds. The shape of the molecule is trigonal planar.
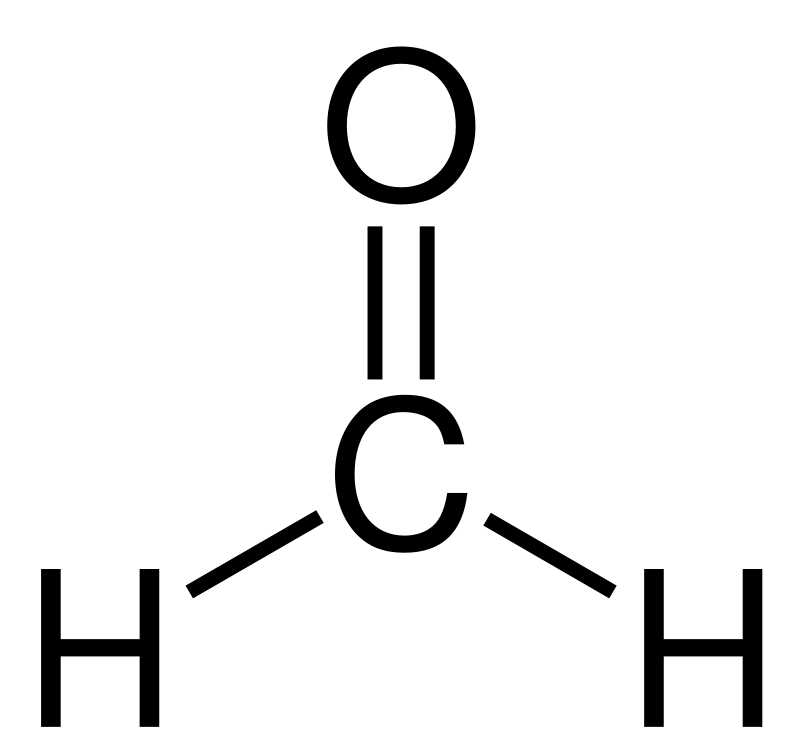
Figure 01: The Chemical Structure of Formaldehyde
An aqueous solution of formaldehyde is flammable and corrosive. When considering the preparation of a formaldehyde solution, we need to add methanol to the reaction mixture in order to prevent formaldehyde from precipitating as paraformaldehyde. In cold conditions, formaldehyde tends to form cloudiness in the solution due to the formation of macromolecules via formaldehyde polymerization.
There are many applications of formaldehyde in industries and other areas. It is used as a precursor for many organic synthesis processes, for example, resins such as melamine resin, phenol-formaldehyde resin. In addition, it is used as a disinfectant. It can kill bacteria and fungi on wood surfaces. However, formaldehyde is toxic and known to be carcinogenic.
What is Acetaldehyde?
Acetaldehyde is an organic compound having the chemical formula CH3CHO, and its IUPAC name is ethanal. This compound contains a methyl group attached to an aldehyde functional group; thus, we can abbreviate it as MeCHO, where Me refers to Methyl. This is an important aldehyde compound that occurs widely in nature; for instance, it occurs naturally in coffee, bread, and ripe fruit. However, it is also produced on a large scale for industrial purposes. Another biological route exists for its preparation; this route involves the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase, and this preparation helps hangovers after alcohol consumption.
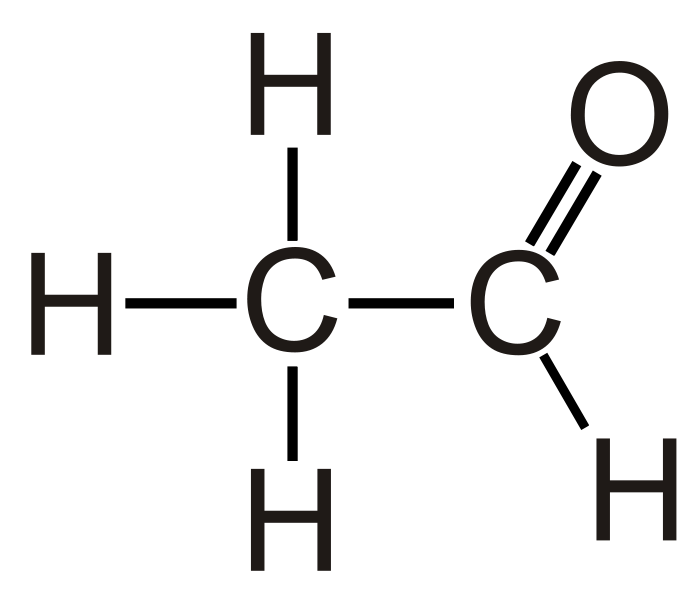
Figure 02: The Chemical Structure of Acetaldehyde
At room temperature and pressure, ethanal occurs as a colorless liquid. Some chemical facts about this substance are as follows:
- Chemical formula is C2H4O
- Molar mass is 44.053 g/mol.
- Appears as a colorless liquid.
- This substance has an ethereal odor.
- The melting point is -123.37 Celsius degrees.
- Boiling point is 20.0 Celsius degrees.
- Miscible with water, ethanol, ether, benzene, toluene, etc.
The molecule has trigonal planar around the carbonyl carbon atom and tetrahedral geometry around the methyl carbon. There are many different uses of ethanal, including its role as a precursor to acetic acid production, as a starting material in the synthesis of 1-butanol, perfumes, flavorings, aniline dyes, plastics, synthetic rubber, etc.
What is the Difference Between Formaldehyde and Acetaldehyde?
Formaldehyde and acetaldehyde are organic compounds we can categorize as aldehyde compounds. The key difference between formaldehyde and acetaldehyde is that formaldehyde contains a hydrogen atom bonded to the carbonyl functional group, whereas acetaldehyde contains a methyl group attached to the carbonyl functional group.
The below infographic presents the differences between formaldehyde and acetaldehyde in tabular form for side by side comparison.
Summary – Formaldehyde vs Acetaldehyde
Formaldehyde is the simplest aldehyde having the chemical formula CH2O. Acetaldehyde is an organic compound having the chemical formula CH3CHO. The key difference between formaldehyde and acetaldehyde is that formaldehyde contains a hydrogen atom bonded to the carbonyl functional group, whereas acetaldehyde contains a methyl group attached to the carbonyl functional group.
Reference:
1. “Acetaldehyde (CH3CHO) – Properties, Structure, Formula & Uses of Acetaldehyde with FAQS.” BYJU’S, 22 Nov. 2021.
Image Courtesy:
1. “Structural formula of formaldehyde” By Chem Sim 2001 – Own work (Public Domain) via Commons Wikimedia
2. “Acetaldehyde-2D-flat” By UAwiki – Own work (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoyfpqulkaGxprTYnZxmmZ6ZeqKvxK2YpZyVncalsY4%3D