What is the Difference Between Ionic Equilibrium and Chemical Equilibrium
The key difference between ionic equilibrium and chemical equilibrium is that ionic equilibrium occurs between unionized molecules and ions in an electrolyte, whereas chemical equilibrium occurs between chemical reactants and products.
Ionic and chemical equilibrium are important phenomena in chemistry. Ionic equilibrium is the equilibrium that is established between unionized molecules and the ions in a solution of weak electrolytes. Chemical equilibrium is the state in which both reactants and products are present in concentrations that have no further tendency to change with time.
CONTENTS
1. Overview and Key Difference
2. What is Ionic Equilibrium
3. What is Chemical Equilibrium
4. Ionic Equilibrium vs Chemical Equilibrium in Tabular Form
5. Summary – Ionic Equilibrium vs Chemical Equilibrium
What is Ionic Equilibrium?
Ionic equilibrium can be described as the equilibrium established between the unionized molecules and the ions in a solution of weak electrolytes. Generally, pH measures the acidity or alkalinity of a solution. This is because acids tend to release hydrogen ions into the solution. If a sparingly soluble salt is dissolved in water, an ionic equilibrium is created.
Ionic equilibrium is also a type of equilibrium where the amounts of products and reactants do not change over time. However, this does not mean that the reaction has stopped; rather, the reaction is proceeding in a way that keeps the amounts unchanged (the net change is zero).
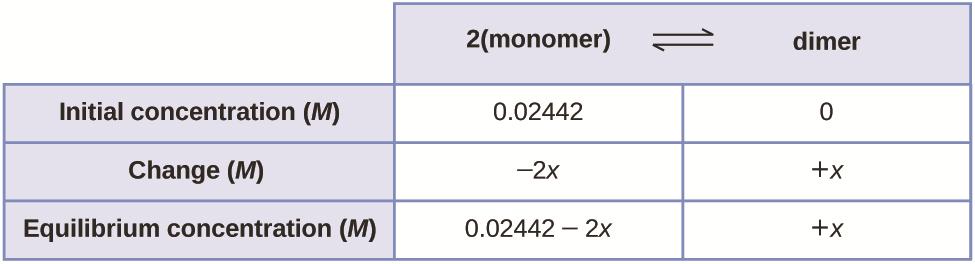
Ionic equilibrium is also known as a “dynamic equilibrium.” In this type of equilibrium, the reaction is reversible and continuing. For a dynamic equilibrium to take place, the system should be a closed one so that no energy or matter escapes from the system.
What is Chemical Equilibrium?
Chemical equilibrium can be described as the state in which both reactants and products are present in concentrations that have no further tendency to change with time. Some reactions are reversible, and some reactions are irreversible. In a reaction, reactants convert to products. In some reactions, reactants are generated again from the products. Hence, this type of reaction is reversible.
In irreversible reactions, once the reactants are converted to products, they do not regenerate again from the products. In a reversible reaction, when reactants are going to products, we call it a forward reaction, and when products are going to reactants, it is a backward reaction.
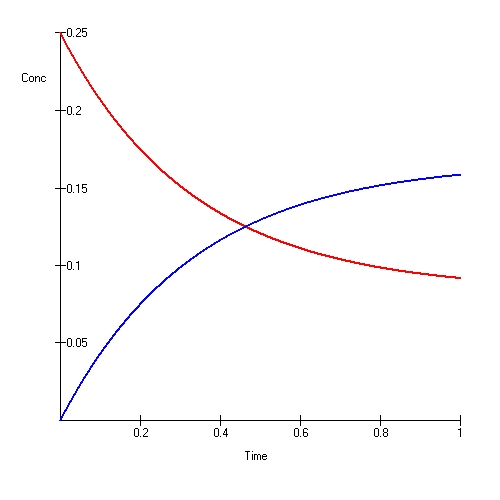
When the rate of forward and backward reactions is equal, then the reaction is at equilibrium. Therefore, over some time, the amount of reactants and products do not change. Reversible reactions always tend to come to equilibrium and maintain that equilibrium. When the system is at an equilibrium, the amount of products and the reactants are not necessarily equal. There can be a higher amount of reactants than products or vice versa. The only requirement in an equilibrium equation is the maintenance of a constant amount from both over time. For a reaction in equilibrium, we can define an equilibrium constant as: where it is equal to the ratio between the concentration of products and the concentration of reactions.
For an equilibrium reaction, if the forward reaction is exothermic, then the backward reaction is endothermic and vice versa. Normally, all the other parameters for forward and backward reactions are opposite to each other like this. Therefore, if we want to facilitate either one of the reactions, we simply have to adjust the parameters to facilitate that reaction.
What is the Difference Between Ionic Equilibrium and Chemical Equilibrium?
Ionic and chemical equilibrium are important phenomena in chemistry. The key difference between ionic equilibrium and chemical equilibrium is that ionic equilibrium occurs between unionized molecules and ions in an electrolyte, whereas chemical equilibrium occurs between chemical reactants and products.
The below infographic presents the differences between ionic equilibrium and chemical equilibrium in tabular form for side by side comparison.
Summary – Ionic Equilibrium vs Chemical Equilibrium
Ionic equilibrium is the equilibrium that is established between the unionized molecules and the ions in a solution of weak electrolytes. Chemical equilibrium is the state in which both reactants and products are present in concentrations that have no further tendency to change with time. The key difference between ionic equilibrium and chemical equilibrium is that ionic equilibrium occurs between unionized molecules and ions in an electrolyte, whereas chemical equilibrium occurs between chemical reactants and products.
Reference:
1. “Introduction, Types and Function of Ionic Equilibrium.” Kullabs.
Image Courtesy:
1. “CNX Chem 13 04 ICETable25 img” By OpenStax – (CC BY 4.0) via Commons Wikimedia
2. “Chemical Equilibrium” By V8rik at the English-language Wikipedia (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoyipqehk2KyssHIpaCbqpmqum6tzZ1knKCVorakrctmnKqtmaG2o77IrqRo