What is the Difference Between Positive and Negative Allosterism
The key difference between positive and negative allosterism is that positive allosterism in proteins shows a high affinity for ligands, whereas negative allosterism in proteins show a low affinity for ligands.
Allosterism or allosteric behavior is the phenomenon in which the activity of a protein can be altered depending on the binding of some molecules at a site other than the active site of the protein (specifically in enzymes). A positive allosterism indicates that the binding of an effector molecule to an enzyme causes the enzyme to change its configuration into an active form. In contrast, a negative allosteirsm indicates that an effector molecule binding causes the enzyme to change its configuration from an active form to an inactive form.
CONTENTS
1. Overview and Key Difference
2. What is Positive Allosterism
3. What is Negative Allosterism
4. Positive vs Negative Allosterism in Tabular Form
5. Summary – Positive vs Negative Allosterism
What is Positive Allosterism?
Positive allosterism is the change in the configuration of a protein (mostly an enzyme) from an inactive form to an active form upon the binding of an effector molecule. The effector molecule binds with a site other than the active site of the enzyme; it is called the allosteric site. This process is also known as allosteric activation.
A common example for such effector molecule binding is the bonding of oxygen molecule with hemoglobin molecule, which activates the hemoglobin molecule to effectively transport oxygen to cells. There, the oxygen molecule binds with the ferrous iron of a heme molecule in the hemoglobin molecule. The active form is known as oxy-hemoglobin, while the inactive form is known as deoxy-hemoglobin.
What is Negative Allosterism?
Negative allosterism is the change in the configuration of an enzyme from an active form to an inactive form upon the binding of an effector molecule. The effector molecule binds with a site other than the active site of the enzyme; it is called the allosteric site. This process is also known as allosteric inhibition.
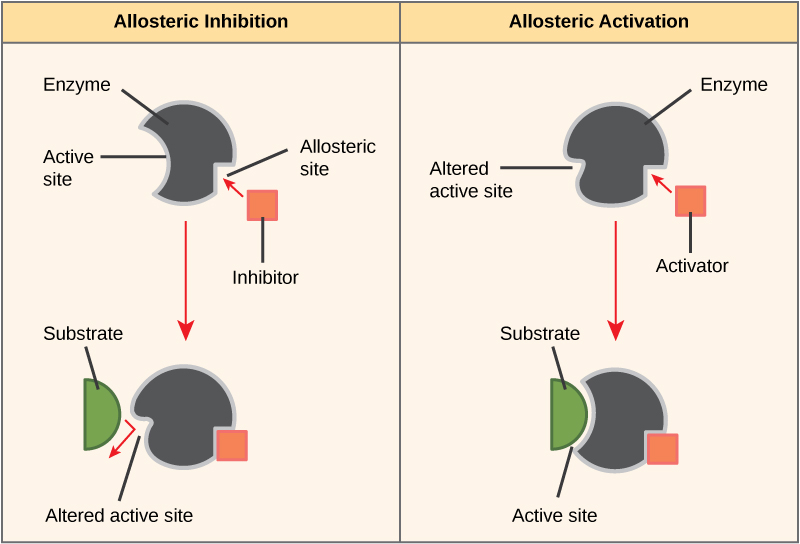
Figure 01: Positive and Negative Allosterism
During negative allosterism, the binding of one ligand decreases the affinity of the enzyme for substrate at the other active sites available for substrate binding. An example is the binding of 2,3-BPG to an allosteric site on hemoglobin, which causes a decrease in the affinity for oxygen of all subunits.
What is the Difference Between Positive and Negative Allosterism?
In a positive allosterism, the binding of an effector molecule to an enzyme causes the enzyme to change its configuration into an active form, while in negative allosteirsm, binding of an effector molecule causes the enzyme to change its configuration from active form to the inactive form. The key difference between positive and negative allosterism is that positive allosterism in proteins shows a high affinity for ligands, whereas negative allosterism in proteins show a low affinity for ligands. In addition, positive allosterism involves activation, whereas negative allosteirsm involves inhibition. Binding of oxygen with hemoglobin is an example of positive allosterism while binding of 2,3-BPG with hemoglobin is an example of negative allosterism.
The following infographic presents the difference between positive and negative allosterism in tabular form for side by side comparison.
Summary – Positive vs Negative Allosterism
In allosterism or allosteric behavior, the activity of a protein can be altered depending on the binding of some molecules at a site other than the active site of the protein (specifically in enzymes). The key difference between positive and negative allosterism is that positive allosterism in proteins shows a high affinity for ligands, whereas negative allosterism in proteins show a low affinity for ligands.
Reference:
1. “3.6: Allosteric Interactions.” Chemistry LibreTexts, Libretexts, 17 July 2020.
Image Courtesy:
2. “Figure 06 05 05 – allosteric activation and inhibition” By CNX OpenStax – (CC BY 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoyppqyhpJ7DpnnAp5tmppWcrrW11Z5kmqScpMC1sdGiqqZn