What is the Difference Between Protic Acid and Lewis Acid
The key difference between protic acid and Lewis acid is that protic acids are proton donors, whereas Lewis acids are proton acceptors.
Protic acids are chemical compounds that can produce a proton or a hydronium ion in their solution by releasing a proton. A Lewis acid is a chemical compound that can accept an electron pair from an electron-donating chemical species.
CONTENTS
1. Overview and Key Difference
2. What is Protic Acid
3. What is Lewis Acid
4. Protic Acid vs Lewis Acid in Tabular Form
5. Summary – Protic Acid vs Lewis Acid
What is Protic Acid?
Protic acids are chemical compounds that can produce a proton or a hydronium ion by releasing a proton. This proton release occurs because these acids can accept a pair of electrons from OH- ion in water by acting as a Lewis acid, but it cannot produce a hydronium ion or a proton by itself.
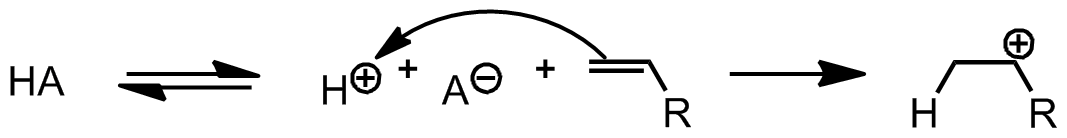
Figure 01: Action of Protic Acids
There are three major types of protic acids as monoprotic acids, polyprotic acids, and diprotic acids . Monoprotic acids can release one proton to the solution, while diprotic acids can release two protons. On the other hand, polyprotic acids can release more than two protons. In polyprotic acids, the protons are released in several steps. However, the first proton is lost from the acid more easily than the next proton.
What is Lewis Acid?
A Lewis acid is a chemical compound that can accept an electron pair from an electron-donating chemical species. This type of acidic compound contains an empty orbital that is able to accept an electron pair from a Lewis base, forming a Lewis adduct. In contrast, Lewis base is a chemical species having a filled orbital consisting of an electron pair. This electron pair does not participate in bonding, but it can form dative bonds with Lewis acids to form a Lewis adduct.
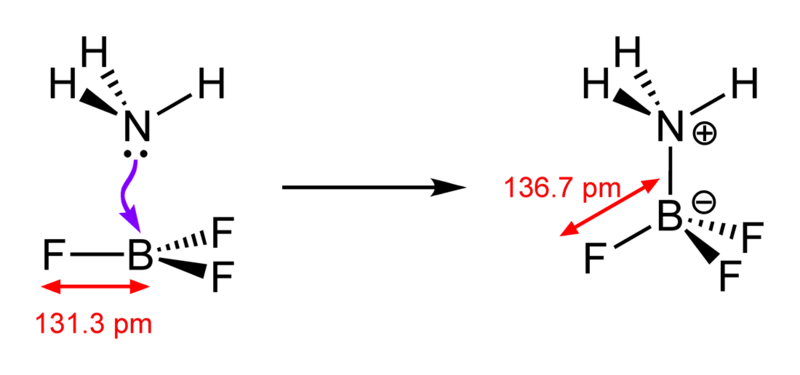
Figure 02: Formation of a Lewis Adduct
Typically, the term Lewis acid is only used with trigonal planar chemical compounds containing an empty p orbital. There, we can treat even complex compounds such as Et3Al2Cl3 as trigonal planar compounds that can be named Lewis acids. Apart from the formation of Lewis adducts, other reactions involving Lewis acids are known as acid-catalyzed reactions. Sometimes, we come across chemical compounds such as H2O having both Lewis acid and Lewis base properties. This is because these compounds can either donate or accept electron pairs, depending on the chemical reaction in which they are involved.
There are various Lewis acids. The simplest Lewis acids tend to react easily and directly with Lewis bases. Most common Lewis acids tend to undergo a chemical reaction prior to the formation of the adduct. Some examples for Lewis acids include onium ions such as ammonium ion and hydronium ion, metal cations such as ferric ion, trigonal planar species such as BF3, electron-poor pi systems such as enones, etc. The three major types of Lewis acids include simple Lewis acids, complex Lewis acids and H+ Lewis acid. The most common application of Lewis acids is the Friedel-Crafts alkylation.
What is the Difference Between Protic Acid and Lewis Acid?
We can distinguish protic acids from Lewis acids through the action of proton release from the acid compound. The key difference between protic acid and Lewis acid is that protic acids are proton donors, whereas Lewis acids are proton acceptors.
The following infographic presents the difference between protic acid and Lewis acid in tabular form.
Summary – Protic Acid vs Lewis Acid
In conclusion, protic acids differ from Lewis acids depending on the ability of these compounds to release protons to the solution. The key difference between protic acid and Lewis acid is that protic acids are proton donors, whereas Lewis acids are proton acceptors.
Reference:
1. “Polyprotic Acids and Bases.” Chemistry LibreTexts, Libretexts, 15 Aug. 2020.
Image Courtesy:
1. “Protic acid initiation” By MatChem121 – Own work (CC BY 3.0) via Commons Wikimedia
2. “NH3-BF3-adduct-bond-lengthening-2D” By Ben Mills – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoypqaismZh6oq%2FInWSappRiuabDyKxkmpuZmXw%3D