What is the Difference Between Silver Nitrate and Silver Sulfadiazine
The key difference between silver nitrate and silver sulfadiazine is that silver nitrate releases a large amount of silver ions at once, whereas silver sulfadiazine releases a steady supply of silver ions over a long time period.
Silver nitrate and silver sulfadiazine are two forms of silver-containing chemical compounds. Silver nitrate is an inorganic compound having the chemical formula AgNO3. Silver sulfadiazine is a topical antibiotic compound useful in partial-thickness and full-thickness burns to prevent infections.
CONTENTS
1. Overview and Key Difference
2. What is Silver Nitrate
3. What is Silver Sulfadiazine
4. Silver Nitrate vs Silver Sulfadiazine in Tabular Form
5. Summary – Silver Nitrate vs Silver Sulfadiazine
What is Silver Nitrate?
Silver nitrate is an inorganic compound having the chemical formula AgNO3. It is a salt of silver that is a versatile precursor to many other compounds of silver. This compound is less sensitive to light compared to halides. The appearance of this compound is as follows.

Figure 01: Silver Nitrate
We can prepare silver nitrate through the reaction of silver with nitric acid. In this reaction, we can use silver in the form of silver bullion or silver foil. This reaction results in silver nitrate, water, and oxides of nitrogen. The formation of the byproducts from this reaction depends mainly on the concentration of nitric acid. Moreover, we need to perform this reaction under a fume hood. This is because this reaction releases toxic nitrogen oxides.
Typically, silver nitrate reacts with a rod of copper in a solution of silver nitrate (leave for few hours), and the silver nitrate compound tends to react with the copper forming hairlike crystals of silver metal. This reaction also gives a blue color solution of copper nitrate.
There are many uses of silver nitrate, including its use as a precursor to other silver compounds, abstraction of halides, synthesis of organic compounds such as deprotection and oxidation reactions, silver staining in histology, etc.
What is Silver Sulfadiazine?
Silver sulfadiazine is a topical antibiotic compound useful in partial-thickness and full-thickness burns to prevent infections. This compound comes under the sulfonamide group. It is a sulfa derivative topical antimicrobial substance useful in 2nd, and 3rd degree burns. It can kill a wide variety of bacteria, but it is sometimes prescribed for some other uses as well.
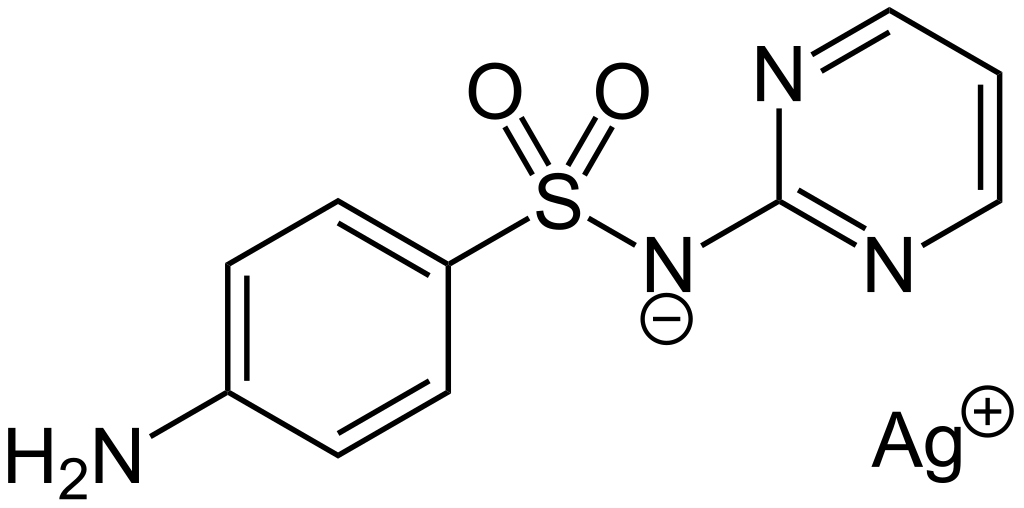
Figure 02: The Chemical Structure of Silver Sulfadiazine
There can be some common side effects of this medication, including itching and pain at the site of application, low white blood cell level, allergic reactions, bluish-grey discolouration, red blood cell break down, liver inflammation, etc.
What is the Difference Between Silver Nitrate and Silver Sulfadiazine?
Silver nitrate and silver sulfadiazine are two forms of silver-containing chemical compounds. The key difference between silver nitrate and silver sulfadiazine is that silver nitrate releases a large amount of silver ions at once, whereas silver sulfadiazine releases a steady supply of silver ions over a long time period. Moreover, there are many different uses of silver nitrate, including its use in photographic films, extraction of halides, production of silver-based explosives, etc., while silver sulfadiazine is mainly useful as a medication to treat burns and infections.
The below infographic lists the differences between silver nitrate and silver sulfadiazine in tabular form for side by side comparison.
Summary – Silver Nitrate vs Silver Sulfadiazine
Silver is a very important chemical element in the periodic table that forms stable chemical compounds having important applications. Silver nitrate and silver sulfadiazine are two forms of silver-containing chemical compounds. The key difference between silver nitrate and silver sulfadiazine is that silver nitrate releases a large amount of silver ions at once, whereas silver sulfadiazine releases a steady supply of silver ions over a long time period.
Reference:
1. “Silver Sulfadiazine.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine.
Image Courtesy:
1. “Silver nitrate crystals” By W. Oelen – (CC BY-SA 3.0) via Commons Wikimedia
2. “Silver sulfadiazine Structural Formula V1” By Jü – Own work (Public Domain) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoysoKWulad6r7XTq5itnV2Wu6V50qKjr52iYsC2uMWam6KZqp67pns%3D