What is the Difference Between Urea and Potassium Sulphate
The key difference between urea and potassium sulphate is their importance as a fertilizer. Urea is important in providing plants with nitrogen, which can promote green leafy growth, whereas potassium sulfate is important in providing plants with potassium and sulfur chemical species.
Urea can be described as an organic compound having the chemical formula CO(NH2)2, while potassium sulfate is an organic compound having the chemical formula K2SO4.
CONTENTS
1. Overview and Key Difference
2. What is Urea
3. What is Potassium Sulphate
4. Urea vs Potassium Sulphate in Tabular Form
5. Summary – Urea vs Potassium Sulphate
What is Urea?
Urea is an organic compound having the chemical formula CO(NH2)2. It is usually known as carbamide, as well. It is a type of amide consisting of two amino groups attached to a central carbonyl carbon atom. Urea molecule is a planar molecule where solid urea contains the oxygen center engaged in two N-H-O hydrogen bonds. The carbon atom in the urea molecule has sp2 hybridization. Further, the C-N bonds of the molecule have a significant double bond character. However, the oxygen atom in the carbonyl group has basicity when compared to formaldehyde. Furthermore, this compound has a high water solubility, which reflects its ability to take part in hydrogen bonding with water molecules.
Typically, urea substances tend to play an important role during the metabolism of nitrogen-consisting compounds in animals, and this compound is the major nitrogen-containing substance in urine passed by animals. It is a colorless, odorless solid substance and has a high-water solubility. Further, it is a nontoxic compound, and when it is dissolved in water, the aqueous solution of urea is neither acidic nor alkaline.
When considering the other uses of urea, it is useful in agriculture and is a component in nitrogen-releasing fertilizers. This is because urea has a high nitrogen content, and it has a low transportation cost per unit nitrogen nutrient. Moreover, urea is important as a raw material for the production of urea-formaldehyde resins and urea-melamine-formaldehyde materials.
What is Potassium Sulphate?
Potassium sulfate (0r sulphate) is an organic compound having the chemical formula K2SO4. It is also known as sulphate of potash or potash of sulfur. It is an inorganic compound that occurs as a white-colored compound that is water-soluble. Most commonly, this substance is used in the production of fertilizers, which can provide both potassium and sulfur atoms to the desired area.

Figure 01: Potassium Sulfate
The natural sources of potassium sulphate include minerals such as kainite, schonite, leonite, langbeinite, and polyhalite. We can separate potassium sulfate from these minerals because the corresponding salt is less water-soluble. Moreover, we can combine kieserite with a solution of potassium chloride to produce potassium sulfate.
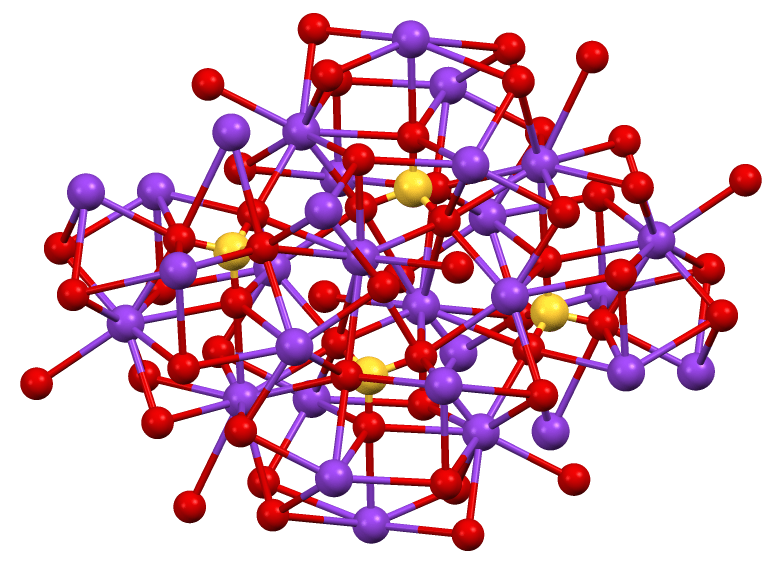
Figure 02: Complex Structure of Beta Potassium Sulfate
There are two types of potassium sulfate as orthorhombic potassium sulfate and tetrahedral potassium sulfate. Among them, the orthorhombic form is commonly known. These are very complex structures. Orthorhombic potassium sulfate converts into alpha potassium sulfate at high temperatures.
What is the Difference Between Urea and Potassium Sulphate?
Urea and potassium sulfate are important substances in agriculture as fertilizers. The key difference between urea and potassium sulphate is that urea, as a fertilizer, is important in providing plants with nitrogen, which can promote green leafy growth, whereas potassium sulfate, as a fertilizer, is important in providing plants with potassium and sulfur chemical species.
Below is a summary of the difference between urea and potassium sulphate in tabular form for side by side comparison.
Summary – Urea vs Potassium Sulphate
Urea can be described as an organic compound having the chemical formula CO(NH2)2. Potassium sulfate is an organic compound having the chemical formula K2SO4. The key difference between urea and potassium sulphate is that urea, as a fertilizer, is important in providing plants with nitrogen, which can promote green leafy growth, whereas potassium sulfate, as a fertilizer, is important in providing plants with potassium and sulfur chemical species.
Reference:
1. “Magnesium Sulfate, Potassium Sulfate, and Sodium Sulfate.” MedlinePlus, U.S. National Library of Medicine.
Image Courtesy:
1. “Arcanite” By Andrew Silver – (Public Domain) via Commons Wikimedia
2. “Structure of K2SO4, K2CrO4 and some related compounds” By Smokefoot – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue9ahmK1lmah6tbTEZpuinpaav6a6wp5km52krLKmuoyuqZ6ZXZa7pXnPqKuaq6Oewq550q6jqaCRqbJw